| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:40:21 UTC |
|---|
| Update Date | 2016-11-09 01:18:45 UTC |
|---|
| Accession Number | CHEM028753 |
|---|
| Identification |
|---|
| Common Name | Isotrichodermin |
|---|
| Class | Small Molecule |
|---|
| Description | From Fusarium culmorum and Fusarium roseum |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
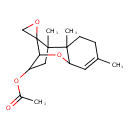
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 12,13-Epoxy-acetate(3alpha)-tichothec-9-en-3-ol | HMDB | | 12,13-Epoxy-trichothec-9-ene-3-ol acetate | HMDB | | 1',2',5'-Trimethyl-8'-oxaspiro[oxirane-2,12'-tricyclo[7.2.1.0²,⁷]dodecan]-5'-en-10'-yl acetic acid | Generator | | Isotrichodermin | MeSH |
|
|---|
| Chemical Formula | C17H24O4 |
|---|
| Average Molecular Mass | 292.370 g/mol |
|---|
| Monoisotopic Mass | 292.167 g/mol |
|---|
| CAS Registry Number | 91423-90-4 |
|---|
| IUPAC Name | 1',2',5'-trimethyl-8'-oxaspiro[oxirane-2,12'-tricyclo[7.2.1.0²,⁷]dodecan]-5'-en-10'-yl acetate |
|---|
| Traditional Name | 1',2',5'-trimethyl-8'-oxaspiro[oxirane-2,12'-tricyclo[7.2.1.0²,⁷]dodecan]-5'-en-10'-yl acetate |
|---|
| SMILES | CC(=O)OC1CC2(C)C3(CO3)C1OC1C=C(C)CCC21C |
|---|
| InChI Identifier | InChI=1S/C17H24O4/c1-10-5-6-15(3)13(7-10)21-14-12(20-11(2)18)8-16(15,4)17(14)9-19-17/h7,12-14H,5-6,8-9H2,1-4H3 |
|---|
| InChI Key | OZXJPPYWZVBMGW-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as trichothecenes. These are sesquiterpene mycotoxins structurally characterized by the presence of an epoxide ring and a benzopyran derivative with a variant number of hydroxyl, acetyl, or other substituents. The most important structural features causing the biological activities of trichothecenes are the 12,13-epoxy ring, the presence of hydroxyl or acetyl groups at appropriate positions on the trichothecene nucleus and the structure and position of the side-chain. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Sesquiterpenoids |
|---|
| Direct Parent | Trichothecenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Trichothecene skeleton
- Oxepane
- Oxane
- Carboxylic acid ester
- Carboxylic acid derivative
- Dialkyl ether
- Oxirane
- Ether
- Monocarboxylic acid or derivatives
- Oxacycle
- Organoheterocyclic compound
- Carbonyl group
- Organooxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organic oxygen compound
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-3890000000-b63210e2a4ef1521c745 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0190000000-288514832dc58420d613 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f7x-2970000000-68774e6875007edb3308 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-7910000000-424d83738d0ac5087690 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-2190000000-af2ee29fea20b34492f5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052e-2190000000-5b8f8e7350dcb7e3b7a1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-5910000000-9f84bec163abe24468bf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0090000000-a2b536561579ef1661d1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000x-0090000000-245aaffd0711e1434dd0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0596-6190000000-ce9dcdcfe902bd55ee9b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0007-0090000000-a7894183665121fad96c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4j-8090000000-c81e27de83898aaf7d30 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9140000000-fb8a6ec5edeb6ebc224f | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0034738 |
|---|
| FooDB ID | FDB013280 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00012645 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 11399530 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 14733836 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|