| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:36:06 UTC |
|---|
| Update Date | 2016-11-09 01:18:44 UTC |
|---|
| Accession Number | CHEM028655 |
|---|
| Identification |
|---|
| Common Name | (4E,9a)-9-(3-Methyl-2E-pentenoyloxy)-4,10(14)-oplopadien-3-one |
|---|
| Class | Small Molecule |
|---|
| Description | (4E,9a)-9-(3-Methyl-2E-pentenoyloxy)-4,10(14)-oplopadien-3-one is found in tea. (4E,9a)-9-(3-Methyl-2E-pentenoyloxy)-4,10(14)-oplopadien-3-one is a constituent of Tussilago farfara (coltsfoot) |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
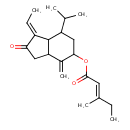
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (1E)-1-Ethylidene-4-methylidene-2-oxo-7-(propan-2-yl)-octahydro-1H-inden-5-yl (2E)-3-methylpent-2-enoic acid | HMDB |
|
|---|
| Chemical Formula | C21H30O3 |
|---|
| Average Molecular Mass | 330.461 g/mol |
|---|
| Monoisotopic Mass | 330.219 g/mol |
|---|
| CAS Registry Number | 237407-01-1 |
|---|
| IUPAC Name | (1E)-1-ethylidene-4-methylidene-2-oxo-7-(propan-2-yl)-octahydro-1H-inden-5-yl (2E)-3-methylpent-2-enoate |
|---|
| Traditional Name | (1E)-1-ethylidene-7-isopropyl-4-methylidene-2-oxo-hexahydroinden-5-yl (2E)-3-methylpent-2-enoate |
|---|
| SMILES | CC\C(C)=C\C(=O)OC1CC(C(C)C)C2C(CC(=O)\C2=C\C)C1=C |
|---|
| InChI Identifier | InChI=1S/C21H30O3/c1-7-13(5)9-20(23)24-19-11-16(12(3)4)21-15(8-2)18(22)10-17(21)14(19)6/h8-9,12,16-17,19,21H,6-7,10-11H2,1-5H3/b13-9+,15-8- |
|---|
| InChI Key | ORVROQPLYIDWBD-VERMXVBFSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as sesquiterpenoids. These are terpenes with three consecutive isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Sesquiterpenoids |
|---|
| Direct Parent | Sesquiterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Sesquiterpenoid
- Oplopane sesquiterpenoid
- Fatty acid ester
- Fatty acyl
- Alpha,beta-unsaturated carboxylic ester
- Enoate ester
- Cyclic ketone
- Ketone
- Carboxylic acid ester
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organic oxide
- Organic oxygen compound
- Organooxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-066v-9451000000-cd7da0f4e63d4eb13dd3 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05o1-9057000000-d9738fada9b6a0a87bef | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0pb9-9110000000-e30c1c7fcbcc39faa4fe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pb9-9300000000-fa2663616971eb3dc7d2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-2059000000-52faae33fe86c577aa0d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-3193000000-33d9f78249940e703a20 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0gc3-9180000000-a1551c505aba05c57d4a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-003r-0197000000-37632b539082636652a0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-02ai-3392000000-cde68c60fd14d1381d56 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-3490000000-817e8f7c7650602244f3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00or-0971000000-06ee1946c0ea2d81e238 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kr-1940000000-6c3ee6c976f1b1765ecb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052o-9410000000-2812805ad3a36f277529 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0034632 |
|---|
| FooDB ID | FDB013154 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35013753 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131751597 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|