| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:35:57 UTC |
|---|
| Update Date | 2016-11-09 01:18:44 UTC |
|---|
| Accession Number | CHEM028652 |
|---|
| Identification |
|---|
| Common Name | (7'R*,8'S*)-Methyl 4,7'-epoxy-3,8'-bilign-7-ene-4',9'-dihydroxy-3',5-dimethoxy-9-oate |
|---|
| Class | Small Molecule |
|---|
| Description | (7'R*,8'S*)-Methyl 4,7'-epoxy-3,8'-bilign-7-ene-4',9'-dihydroxy-3',5-dimethoxy-9-oate is found in fruits. (7'R*,8'S*)-Methyl 4,7'-epoxy-3,8'-bilign-7-ene-4',9'-dihydroxy-3',5-dimethoxy-9-oate is a constituent of Zizyphus jujuba (Chinese date) |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
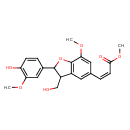
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (7'R*,8's*)-methyl 4,7'-epoxy-3,8'-bilign-7-ene-4',9'-dihydroxy-3',5-dimethoxy-9-Oic acid | Generator | | Methyl (2Z)-3-[2-(4-hydroxy-3-methoxyphenyl)-3-(hydroxymethyl)-7-methoxy-2,3-dihydro-1-benzofuran-5-yl]prop-2-enoic acid | HMDB |
|
|---|
| Chemical Formula | C21H22O7 |
|---|
| Average Molecular Mass | 386.395 g/mol |
|---|
| Monoisotopic Mass | 386.137 g/mol |
|---|
| CAS Registry Number | 107296-48-0 |
|---|
| IUPAC Name | methyl (2Z)-3-[2-(4-hydroxy-3-methoxyphenyl)-3-(hydroxymethyl)-7-methoxy-2,3-dihydro-1-benzofuran-5-yl]prop-2-enoate |
|---|
| Traditional Name | methyl (2Z)-3-[2-(4-hydroxy-3-methoxyphenyl)-3-(hydroxymethyl)-7-methoxy-2,3-dihydro-1-benzofuran-5-yl]prop-2-enoate |
|---|
| SMILES | COC(=O)\C=C/C1=CC2=C(OC(C2CO)C2=CC(OC)=C(O)C=C2)C(OC)=C1 |
|---|
| InChI Identifier | InChI=1S/C21H22O7/c1-25-17-10-13(5-6-16(17)23)20-15(11-22)14-8-12(4-7-19(24)27-3)9-18(26-2)21(14)28-20/h4-10,15,20,22-23H,11H2,1-3H3/b7-4- |
|---|
| InChI Key | DPDJZJNYBSGDHT-DAXSKMNVSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 2-arylbenzofuran flavonoids. These are phenylpropanoids containing the 2-phenylbenzofuran moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | 2-arylbenzofuran flavonoids |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | 2-arylbenzofuran flavonoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - 2-arylbenzofuran flavonoid
- Neolignan skeleton
- Coumaric acid or derivatives
- Methoxyphenol
- Coumaran
- Benzofuran
- Phenoxy compound
- Anisole
- Methoxybenzene
- Phenol ether
- Styrene
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Phenol
- Fatty acid ester
- Monocyclic benzene moiety
- Fatty acyl
- Benzenoid
- Alpha,beta-unsaturated carboxylic ester
- Enoate ester
- Methyl ester
- Carboxylic acid ester
- Ether
- Organoheterocyclic compound
- Carboxylic acid derivative
- Oxacycle
- Monocarboxylic acid or derivatives
- Organooxygen compound
- Primary alcohol
- Alcohol
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-0009000000-dfed748fc1fd4b76de91 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-066r-1000960000-dc2e5da8f72315d7d1e9 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05n0-0009000000-9f34f4537f3b798ed875 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-07br-0129000000-2d1db0eff775f27824d1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fb9-1911000000-3d48f8e9f1009e470d01 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0009000000-4351ef3bd5e009175ddc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0009000000-83b06152873a3b6e6218 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0079-2239000000-5d5b64c9d5f8d607928a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052r-0009000000-02adff729b41d95d0577 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05p9-0019000000-c12011103cd094f14897 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-0938000000-314cd77e015d2466f7ac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-002r-0009000000-5ec7c1fd7f90fa688e44 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0029000000-0065f53f0ce0ee3fa52d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0gx9-0297000000-c62e622f30483cd79707 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0034629 |
|---|
| FooDB ID | FDB013151 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35013750 |
|---|
| ChEBI ID | 173284 |
|---|
| PubChem Compound ID | 131751594 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|