| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:33:58 UTC |
|---|
| Update Date | 2016-11-09 01:18:43 UTC |
|---|
| Accession Number | CHEM028599 |
|---|
| Identification |
|---|
| Common Name | Phenethyl 6-galloylglucoside |
|---|
| Class | Small Molecule |
|---|
| Description | Phenethyl 6-galloylglucoside is found in green vegetables. Phenethyl 6-galloylglucoside is isolated from Rosa damascena (damask rose |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
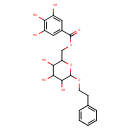
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| [3,4,5-Trihydroxy-6-(2-phenylethoxy)oxan-2-yl]methyl 3,4,5-trihydroxybenzoic acid | HMDB |
|
|---|
| Chemical Formula | C21H24O10 |
|---|
| Average Molecular Mass | 436.409 g/mol |
|---|
| Monoisotopic Mass | 436.137 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | [3,4,5-trihydroxy-6-(2-phenylethoxy)oxan-2-yl]methyl 3,4,5-trihydroxybenzoate |
|---|
| Traditional Name | [3,4,5-trihydroxy-6-(2-phenylethoxy)oxan-2-yl]methyl 3,4,5-trihydroxybenzoate |
|---|
| SMILES | OC1C(O)C(COC(=O)C2=CC(O)=C(O)C(O)=C2)OC(OCCC2=CC=CC=C2)C1O |
|---|
| InChI Identifier | InChI=1S/C21H24O10/c22-13-8-12(9-14(23)16(13)24)20(28)30-10-15-17(25)18(26)19(27)21(31-15)29-7-6-11-4-2-1-3-5-11/h1-5,8-9,15,17-19,21-27H,6-7,10H2 |
|---|
| InChI Key | ZMYPTBCSUJAIQL-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as galloyl esters. These are organic compounds that contain an ester derivative of 3,4,5-trihydroxybenzoic acid. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzoic acids and derivatives |
|---|
| Direct Parent | Galloyl esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Galloyl ester
- P-hydroxybenzoic acid alkyl ester
- M-hydroxybenzoic acid ester
- P-hydroxybenzoic acid ester
- Glycosyl compound
- O-glycosyl compound
- Benzoate ester
- Benzenetriol
- Pyrogallol derivative
- Benzoyl
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Monosaccharide
- Oxane
- Carboxylic acid ester
- Secondary alcohol
- Organoheterocyclic compound
- Monocarboxylic acid or derivatives
- Oxacycle
- Acetal
- Carboxylic acid derivative
- Polyol
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Organooxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0uxr-5892200000-c4438c4cc94b7924b913 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-000i-7484249000-0f8ea00a294deece101b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0gbi-0940700000-33adabc3f8b297e85e45 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ab9-0900000000-9fd4bb6984c5435a9121 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0zi0-1900000000-b39c4ba65bff49671d57 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014r-1911400000-61673ecc9b049114f531 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-1900000000-9b948823b2d13586dd30 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-3900000000-463ee0c950741dfcdb2d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0l0i-1901400000-6cd69271fae557f3633c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0pwi-1924100000-898803fd3111a3b62002 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pb9-1900000000-b2bd25c2310797486167 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03e9-0319000000-07850b2919f47d5d6c2a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-5921000000-427b7c08a5ac9c2b51b9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00ou-4901000000-a09d2021217ed64ac2b2 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0034573 |
|---|
| FooDB ID | FDB013086 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 411427 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 468285 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|