| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:30:58 UTC |
|---|
| Update Date | 2016-11-09 01:18:42 UTC |
|---|
| Accession Number | CHEM028519 |
|---|
| Identification |
|---|
| Common Name | Acevaltrate |
|---|
| Class | Small Molecule |
|---|
| Description | Acevaltrate is found in fats and oils. Acevaltrate is produced by Valeriana specie |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
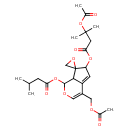
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Acevaltric acid | Generator | | (Acetyloxy)valepotriate | HMDB | | Acetovaltrate | HMDB | | Acetoxyvalepotriate | HMDB | | Acetoxyvaltrate | HMDB | | Acevaltrat | HMDB | | Acevaltrate, inn | HMDB | | Acevaltrato | HMDB | | Acevaltratum | HMDB |
|
|---|
| Chemical Formula | C24H32O10 |
|---|
| Average Molecular Mass | 480.505 g/mol |
|---|
| Monoisotopic Mass | 480.200 g/mol |
|---|
| CAS Registry Number | 25161-41-5 |
|---|
| IUPAC Name | 4-[(acetyloxy)methyl]-1-[(3-methylbutanoyl)oxy]-6,7a-dihydro-1H-spiro[cyclopenta[c]pyran-7,2'-oxirane]-6-yl 3-(acetyloxy)-3-methylbutanoate |
|---|
| Traditional Name | acevaltrate |
|---|
| SMILES | CC(C)CC(=O)OC1OC=C(COC(C)=O)C2=CC(OC(=O)CC(C)(C)OC(C)=O)C3(CO3)C12 |
|---|
| InChI Identifier | InChI=1S/C24H32O10/c1-13(2)7-19(27)33-22-21-17(16(11-30-22)10-29-14(3)25)8-18(24(21)12-31-24)32-20(28)9-23(5,6)34-15(4)26/h8,11,13,18,21-22H,7,9-10,12H2,1-6H3 |
|---|
| InChI Key | FWKBQAVMKVZEOT-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tetracarboxylic acids and derivatives. These are carboxylic acids containing exactly four carboxyl groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Tetracarboxylic acids and derivatives |
|---|
| Direct Parent | Tetracarboxylic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tetracarboxylic acid or derivatives
- Iridoid-skeleton
- Bicyclic monoterpenoid
- Monoterpenoid
- Fatty acid ester
- Fatty acyl
- Carboxylic acid ester
- Oxacycle
- Ether
- Oxirane
- Dialkyl ether
- Acetal
- Organoheterocyclic compound
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Organic oxygen compound
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9557600000-d130463ccdd2dd29b929 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0079-7405900000-4423f1528fe4218d0aed | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000l-9303100000-bee601821263b4473235 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052u-9343000000-d6e10546c6864e44a77e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-9102400000-f4ca5ffec3671bfac3ec | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-053i-9217200000-af850e2673f91c130c64 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a59-9102000000-152355387ea0bdb8520a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0019500000-cc102aa1aab2a8a0b8f9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052o-6029300000-4c15187f741229de7542 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00ko-9028100000-787b1cab1bda1a12c7a2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-002r-2009200000-2165e7cee1aba7002c49 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4s-8009100000-b25b49454bc8ba908cc3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9003000000-bc20b6788e447d4e3bd2 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0034494 |
|---|
| FooDB ID | FDB012987 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00010724 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Acevaltrate |
|---|
| Chemspider ID | 11262880 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 22245467 |
|---|
| Kegg Compound ID | C16752 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|