| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:30:07 UTC |
|---|
| Update Date | 2016-11-09 01:18:42 UTC |
|---|
| Accession Number | CHEM028497 |
|---|
| Identification |
|---|
| Common Name | Austin |
|---|
| Class | Small Molecule |
|---|
| Description | Mycotoxin production by the food storage mould (Aspergillus ustus). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
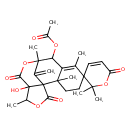
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 12'-Hydroxy-2,2,2',6',9',13'-hexamethyl-16'-methylidene-6,11',15'-trioxo-2,6-dihydro-10',14'-dioxaspiro[pyran-3,5'-tetracyclo[7.6.1.0¹,¹².0²,⁷]hexadecan]-6'-en-8'-yl acetic acid | Generator | | Austin | MeSH |
|
|---|
| Chemical Formula | C27H32O9 |
|---|
| Average Molecular Mass | 500.538 g/mol |
|---|
| Monoisotopic Mass | 500.205 g/mol |
|---|
| CAS Registry Number | 61103-89-7 |
|---|
| IUPAC Name | 12'-hydroxy-2,2,2',6',9',13'-hexamethyl-16'-methylidene-6,11',15'-trioxo-2,6-dihydro-10',14'-dioxaspiro[pyran-3,5'-tetracyclo[7.6.1.0¹,¹².0²,⁷]hexadecan]-6'-en-8'-yl acetate |
|---|
| Traditional Name | 12'-hydroxy-2,2,2',6',9',13'-hexamethyl-16'-methylidene-6,11',15'-trioxo-10',14'-dioxaspiro[pyran-3,5'-tetracyclo[7.6.1.0¹,¹².0²,⁷]hexadecan]-6'-en-8'-yl acetate |
|---|
| SMILES | CC1OC(=O)C23C(=C)C(C)(OC(=O)C12O)C(OC(C)=O)C1=C(C)C2(CCC31C)C=CC(=O)OC2(C)C |
|---|
| InChI Identifier | InChI=1S/C27H32O9/c1-13-18-19(34-16(4)28)24(8)14(2)26(20(30)33-15(3)27(26,32)21(31)36-24)23(18,7)11-12-25(13)10-9-17(29)35-22(25,5)6/h9-10,15,19,32H,2,11-12H2,1,3-8H3 |
|---|
| InChI Key | DEMDOYQPCDXCEB-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tetracarboxylic acids and derivatives. These are carboxylic acids containing exactly four carboxyl groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Tetracarboxylic acids and derivatives |
|---|
| Direct Parent | Tetracarboxylic acids and derivatives |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | Not Available |
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0673-2092700000-6baa8e5f3ca3acac27cf | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-05du-4060290000-8a6be42948caecebf201 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0zfu-0001920000-89f8aea48c17d798ee27 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-054x-0004900000-314a8f350d4e20951320 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-002g-7209400000-cb03b167e6aba8d65589 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4j-1000900000-ed9e8c1c51591467d000 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-2000900000-1115678b470b35578832 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0bt9-8001900000-241fc1259c3ad9435395 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0000690000-173f17c6a52e45df0e07 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0pb9-0002910000-b4162ee2b29e421b35fd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udv-5905300000-e74b33d57cc7462db9eb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000b-1000900000-7f5f3c2fdac9468bd33f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-9000000000-1fba8bc1e1f4f04c2af9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9000000000-b1729fe95ee43ef528e7 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0034463 |
|---|
| FooDB ID | FDB012872 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Austin, Texas |
|---|
| Chemspider ID | 380841 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 430632 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|