| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:28:36 UTC |
|---|
| Update Date | 2016-11-09 01:18:41 UTC |
|---|
| Accession Number | CHEM028462 |
|---|
| Identification |
|---|
| Common Name | Phaseollinisoflavan |
|---|
| Class | Small Molecule |
|---|
| Description | Phaseollinisoflavan is found in common bean. Phytoalexin from Phaseolus vulgaris (kidney bean), other Phaseolus species and Glycyrrhiza glabra (licorice |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
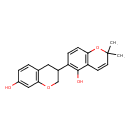
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (-)-Phaseollinisoflavan | HMDB | | 3,4-dihydro-2,2-Dimethyl[3,6'-bi-2H-1-benzopyran]-5',7-diol, 9ci | HMDB | | Phaseolinisoflavan | HMDB | | Phaseollin | MeSH, HMDB | | Phaseollinisoflavan | MeSH |
|
|---|
| Chemical Formula | C20H20O4 |
|---|
| Average Molecular Mass | 324.370 g/mol |
|---|
| Monoisotopic Mass | 324.136 g/mol |
|---|
| CAS Registry Number | 40323-57-7 |
|---|
| IUPAC Name | 6-(7-hydroxy-3,4-dihydro-2H-1-benzopyran-3-yl)-2,2-dimethyl-2H-chromen-5-ol |
|---|
| Traditional Name | 6-(7-hydroxy-3,4-dihydro-2H-1-benzopyran-3-yl)-2,2-dimethylchromen-5-ol |
|---|
| SMILES | CC1(C)OC2=C(C=C1)C(O)=C(C=C2)C1COC2=C(C1)C=CC(O)=C2 |
|---|
| InChI Identifier | InChI=1S/C20H20O4/c1-20(2)8-7-16-17(24-20)6-5-15(19(16)22)13-9-12-3-4-14(21)10-18(12)23-11-13/h3-8,10,13,21-22H,9,11H2,1-2H3 |
|---|
| InChI Key | UUJBHSNXZMGYBT-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyranoisoflavonoids. These are isoflavonoids that contain a pyran ring fused to either of the A, B, or C ring of the isoflavonoid skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Isoflavonoids |
|---|
| Sub Class | Pyranoisoflavonoids |
|---|
| Direct Parent | Pyranoisoflavonoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyranoisoflavonoid
- Hydroxyisoflavonoid
- Isoflavanol
- Isoflavan
- 2,2-dimethyl-1-benzopyran
- Chromane
- Benzopyran
- 1-benzopyran
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Benzenoid
- Oxacycle
- Ether
- Organoheterocyclic compound
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4r-1987000000-8371fc805be7cb9e958c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0zfs-4195600000-664b19f41205127907c4 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00b9-1928000000-ee1050210e0496a816e5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0963000000-424c3aadc1084880ac94 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05fs-3920000000-a53a1bcd304489c7e7b0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0219000000-90765added0f691569a6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00fr-0946000000-0dc59da88ccd590aa271 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05fr-1920000000-9d3bdb7ff48329efe9ec | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0609000000-e1d84e643a7f3ef9fcd0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0869000000-6c445948a577371f6fa1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000b-1931000000-86ca55e080ce88266647 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0009000000-b64caba2598a2db6662e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0139000000-d7b358e9b93d1ccbc2db | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-08gi-0792000000-b48aed48d6345a8de3bc | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0034411 |
|---|
| FooDB ID | FDB012805 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00002560 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 3682779 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 4484952 |
|---|
| Kegg Compound ID | C10515 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|