| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:25:39 UTC |
|---|
| Update Date | 2016-11-09 01:18:41 UTC |
|---|
| Accession Number | CHEM028394 |
|---|
| Identification |
|---|
| Common Name | Physalin M |
|---|
| Class | Small Molecule |
|---|
| Description | A hydroxycoumarin that is umbelliferone bearing a methoxy substituent at position 6. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
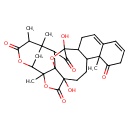
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 6-Methoxy-7-hydroxycoumarin | ChEBI | | 6-Methylesculetin | ChEBI | | 6-O-Methylesculetin | ChEBI | | 7-Hydroxy-6-methoxy-2H-1-benzopyran-2-one | ChEBI | | 7-Hydroxy-6-methoxycoumarin | ChEBI | | 7-Hydroxy-6-methoxy-2H-chromen-2-one | Kegg | | 2H-1-Benzopyran-2-one, 7-hydroxy-6-methoxy- (9ci) | HMDB | | 6-Methoxyumbelliferone | HMDB | | 7-Hydroxy-5-methoxycoumarin | HMDB | | 7-Hydroxy-6-methoxy-coumarin | HMDB | | Acid, chrysotropic | HMDB | | Acid, gelseminic | HMDB | | Aesculetin 6-methyl ether | HMDB | | b-Methylaesculetin | HMDB | | Baogongteng b | HMDB | | beta -Methylesculetin | HMDB | | beta-Methylesculetin | HMDB | | Buxuletin | HMDB | | Chrysatropic acid | HMDB | | Chrysotropic acid | HMDB | | Escopoletin | HMDB | | Esculetin 6-methyl ether | HMDB | | Esculetin-6-methyl ether | HMDB | | Gelseminic acid | HMDB | | Methylesculetin | HMDB | | Murrayetin | HMDB | | Scopoletine | HMDB | | Scopoletol | HMDB |
|
|---|
| Chemical Formula | C28H32O9 |
|---|
| Average Molecular Mass | 512.548 g/mol |
|---|
| Monoisotopic Mass | 512.205 g/mol |
|---|
| CAS Registry Number | 117591-92-1 |
|---|
| IUPAC Name | 5,18-dihydroxy-1,14,21,25-tetramethyl-4,20,23-trioxaheptacyclo[20.3.1.1²,⁵.0³,¹⁸.0³,²¹.0⁶,¹⁵.0⁹,¹⁴]heptacosa-8,10-diene-13,19,24,27-tetrone |
|---|
| Traditional Name | 5,18-dihydroxy-1,14,21,25-tetramethyl-4,20,23-trioxaheptacyclo[20.3.1.1²,⁵.0³,¹⁸.0³,²¹.0⁶,¹⁵.0⁹,¹⁴]heptacosa-8,10-diene-13,19,24,27-tetrone |
|---|
| SMILES | CC1C(=O)OC2CC1(C)C1C(=O)C3(O)OC11C2(C)OC(=O)C1(O)CCC1C3CC=C2C=CCC(=O)C12C |
|---|
| InChI Identifier | InChI=1S/C28H32O9/c1-13-21(31)35-18-12-23(13,2)19-20(30)27(34)16-9-8-14-6-5-7-17(29)24(14,3)15(16)10-11-26(33)22(32)36-25(18,4)28(19,26)37-27/h5-6,8,13,15-16,18-19,33-34H,7,9-12H2,1-4H3 |
|---|
| InChI Key | DRSSQOIGUIMEGX-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 7-hydroxycoumarins. These are coumarins that contain one or more hydroxyl groups attached to the C7 position the coumarin skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Coumarins and derivatives |
|---|
| Sub Class | Hydroxycoumarins |
|---|
| Direct Parent | 7-hydroxycoumarins |
|---|
| Alternative Parents | |
|---|
| Substituents | - 7-hydroxycoumarin
- Benzopyran
- 1-benzopyran
- Anisole
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Pyranone
- Pyran
- Benzenoid
- Heteroaromatic compound
- Lactone
- Oxacycle
- Ether
- Organoheterocyclic compound
- Organic oxygen compound
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00di-0900300000-a66478e795659ea30e2f | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-03mu-3900005000-adf8a60b3addceeafd50 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0000970000-5b65aabce53349bbccbe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01ot-0000910000-749e49787ccb89e8387f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000f-2002900000-e85a5d4179af0192fc8a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0000690000-88350bbc3bf9c341b775 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03xr-0000950000-271032ec5b4884cce89a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0079-1900300000-92c31cbe835c084c94cb | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0034344 |
|---|
| FooDB ID | FDB012705 |
|---|
| Phenol Explorer ID | 638 |
|---|
| KNApSAcK ID | C00002499 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | SCOPOLETIN |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Scopoletin |
|---|
| Chemspider ID | 4444113 |
|---|
| ChEBI ID | 17488 |
|---|
| PubChem Compound ID | 5280460 |
|---|
| Kegg Compound ID | C01752 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | M2MDB005634 |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|