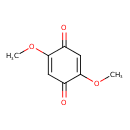
| 2,5-Dimethoxy-4-benzoquinone | MeSH |
| 1,1',1'',1'''-[dithiobis(carbonothioylnitrilo)]tetraethane | HMDB |
| 1,1'-Dithiobis(N,N-diethylthio)-formamide | HMDB |
| 1,1'-Dithiobis(N,N-diethylthio-formamide | HMDB |
| 1,1'-Dithiobis(N,N-diethylthioformamide) | HMDB |
| 1,1'-Dithiobis[N,N-diethylthioformamide] | HMDB |
| 2,5-Dimethoxy-1,4-benzoquinone | HMDB |
| Abstenisil | HMDB |
| Abstensil | HMDB |
| Abstinil | HMDB |
| Abstinyl | HMDB |
| Accel tet | HMDB |
| Accel tet-R | HMDB |
| Alcophobin | HMDB |
| Alk-aubs | HMDB |
| Allphar brand OF disulfiram | HMDB |
| Altana pharma brand OF disulfiram | HMDB |
| Antab use | HMDB |
| Antabus | HMDB |
| Antabuse | HMDB |
| Antadix | HMDB |
| Antaenyl | HMDB |
| Antaethan | HMDB |
| Antaethyl | HMDB |
| Antaetil | HMDB |
| Antalcol | HMDB |
| Antetan | HMDB |
| Antethyl | HMDB |
| Antetil | HMDB |
| Anteyl | HMDB |
| Anthethyl | HMDB |
| Anti-ethyl | HMDB |
| Antiaethan | HMDB |
| Anticol | HMDB |
| Antietanol | HMDB |
| Antiethanol | HMDB |
| Antietil | HMDB |
| Antikol | HMDB |
| Antivitium | HMDB |
| Antivitium (spain) | HMDB |
| Artu brand OF disulfiram | HMDB |
| Aversan | HMDB |
| Averzan | HMDB |
| Bis((diethylamino)thioxomethyl) disulfide | HMDB |
| Bis((diethylamino)thioxomethyl)disulfide | HMDB |
| Bis((diethylamino)thioxomethyl)disulphide | HMDB |
| Bis(diethylthiocarbamoyl) disulfide | HMDB |
| Bis(diethylthiocarbamoyl)disulphide | HMDB |
| Bis(diethylthiocarbamyl) disulfide | HMDB |
| Bis(N,N-diethylthiocarbamoyl) disulfide | HMDB |
| Bis(N,N-diethylthiocarbamoyl)disulphide | HMDB |
| Bis-(diethylthiocarbamoyl)disulfide | HMDB |
| Bis[(diethylamino)thioxomethyl] disulfide | HMDB |
| Bohm brand OF disulfiram | HMDB |
| Bonibal | HMDB |
| Contralin | HMDB |
| Contrapot | HMDB |
| Cronetal | HMDB |
| Dicupral | HMDB |
| Diethylcarbamothioylsulfanyl diethylaminomethanedithioate | HMDB |
| Disetil | HMDB |
| Disulfamide | HMDB |
| Disulfan | HMDB |
| Disulfide, bis(diethylthiocarbamoyl) | HMDB |
| Disulfide, tetraethylthiuram | HMDB |
| Disulfiram | HMDB |
| Disulfiram (JP15/usp/inn) | HMDB |
| Disulfiram (tetraethylthiuram disulfide) | HMDB |
| Disulfirame | HMDB |
| Disulfiramo | HMDB |
| Disulfiramum | HMDB |
| Disulfirm | HMDB |
| Disulfuram | HMDB |
| Disulphuram | HMDB |
| Dumex brand OF disulfiram | HMDB |
| Dupont fungicide 4472 | HMDB |
| Ekagom dtet | HMDB |
| Ekagom teds | HMDB |
| Ekagom tetds | HMDB |
| Ephorran | HMDB |
| Espenal | HMDB |
| Esperal | HMDB |
| eta Bus | HMDB |
| Etabus | HMDB |
| Ethyl thiram | HMDB |
| Ethyl thiudad | HMDB |
| Ethyl thiurad | HMDB |
| Ethyl tuads | HMDB |
| Ethyl tuex | HMDB |
| Ethyldithiourame | HMDB |
| Ethyldithiurame | HMDB |
| Exhoran | HMDB |
| Exhorran | HMDB |
| HOCA | HMDB |
| Krotenal | HMDB |
| N,N,N',n'-tetraethylthiuram disulfide | HMDB |
| N,N,N',n'-tetraethylthiuram disulphide | HMDB |
| Nocbin | HMDB |
| Nocceler | HMDB |
| Nocceler tet | HMDB |
| Nocceler tet-g | HMDB |
| Noxal | HMDB |
| Odyssey brand OF disulfiram | HMDB |
| Orphan brand OF disulfiram | HMDB |
| Refusal | HMDB |
| ro-Sulfiram | HMDB |
| ro-Sulfram-500 (usa) | HMDB |
| Robac tet | HMDB |
| Sanceler tet | HMDB |
| Sanceler tet-g | HMDB |
| Sanofi synthelabo brand OF disulfiram | HMDB |
| Soxinol tet | HMDB |
| Stopaethyl | HMDB |
| Stopethyl | HMDB |
| Stopety | HMDB |
| Stopetyl | HMDB |
| TATD | HMDB |
| Tenurid | HMDB |
| Tenutex | HMDB |
| Tet raethylthiuram | HMDB |
| TETD | HMDB |
| Tetidis | HMDB |
| Tetradin | HMDB |
| Tetradine | HMDB |
| Tetraethyl-thioperoxydicarbonic diamide | HMDB |
| Tetraethyl-thioperoxydicarbonic diamide (((H2N)C(S))2S2) | HMDB |
| Tetraethyl-thioperoxydicarbonic diamide ((H2N)C(S))2S2 | HMDB |
| Tetraethyl-thioperoxydicarbonic diamide ([(H2N)C(S)]2S2) | HMDB |
| Tetraethyl-thiuram disulfide | HMDB |
| Tetraethylthioperoxydicarbonic diamide | HMDB |
| Tetraethylthioperoxydicarbonic diamide, ((H2N)C(S))2S2 | HMDB |
| Tetraethylthioperoxydicarbonothioic diamide | HMDB |
| Tetraethylthiram disulfide | HMDB |
| Tetraethylthiram disulphide | HMDB |
| Tetraethylthiuram | HMDB |
| Tetraethylthiuram disulfide | HMDB |
| Tetraethylthiuram disulphide | HMDB |
| Tetraethylthiuram sulfide | HMDB |
| Tetraethylthiuran disulfide | HMDB |
| Tetraethylthiurium disulfide | HMDB |
| Tetraetil | HMDB |
| Teturam | HMDB |
| Teturamin | HMDB |
| Thermophyllin | HMDB |
| Thiocid | HMDB |
| Thiosan | HMDB |
| Thioscabin | HMDB |
| Thireranide | HMDB |
| Thiuram e | HMDB |
| Thiuranide | HMDB |
| Tillram | HMDB |
| Tiuram | HMDB |
| TTD | HMDB |
| TTS | HMDB |
| Tuads, ethyl | HMDB |