| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:25:05 UTC |
|---|
| Update Date | 2016-11-09 01:18:40 UTC |
|---|
| Accession Number | CHEM028381 |
|---|
| Identification |
|---|
| Common Name | (3beta,5alpha,6a)-Cholesta-8,14-diene-3,6-diol |
|---|
| Class | Small Molecule |
|---|
| Description | (3beta,5alpha,6a)-Cholesta-8,14-diene-3,6-diol is found in fruits. (3beta,5alpha,6a)-Cholesta-8,14-diene-3,6-diol is a constituent of Stenocereus thurberi (organ pipe cactus) |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
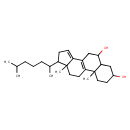
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (3b,5a,6a)-Cholesta-8,14-diene-3,6-diol | Generator | | (3Β,5α,6a)-cholesta-8,14-diene-3,6-diol | Generator | | Thurberol | HMDB |
|
|---|
| Chemical Formula | C27H44O2 |
|---|
| Average Molecular Mass | 400.637 g/mol |
|---|
| Monoisotopic Mass | 400.334 g/mol |
|---|
| CAS Registry Number | 84765-66-2 |
|---|
| IUPAC Name | 2,15-dimethyl-14-(6-methylheptan-2-yl)tetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadeca-1(10),11-diene-5,8-diol |
|---|
| Traditional Name | 2,15-dimethyl-14-(6-methylheptan-2-yl)tetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadeca-1(10),11-diene-5,8-diol |
|---|
| SMILES | CC(C)CCCC(C)C1CC=C2C3=C(CCC12C)C1(C)CCC(O)CC1C(O)C3 |
|---|
| InChI Identifier | InChI=1S/C27H44O2/c1-17(2)7-6-8-18(3)21-9-10-22-20-16-25(29)24-15-19(28)11-13-27(24,5)23(20)12-14-26(21,22)4/h10,17-19,21,24-25,28-29H,6-9,11-16H2,1-5H3 |
|---|
| InChI Key | CZXLHIZQQUJTND-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as cholesterols and derivatives. Cholesterols and derivatives are compounds containing a 3-hydroxylated cholestane core. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Cholestane steroids |
|---|
| Direct Parent | Cholesterols and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cholesterol-skeleton
- Hydroxysteroid
- 6-hydroxysteroid
- 3-hydroxysteroid
- Cyclic alcohol
- Secondary alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-052o-1119000000-c130da4e779e1f7fd64b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0059-4101590000-feafa20ba127b9e03dce | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f89-0009200000-18b2b491bcf0d96e8969 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00lr-3229100000-15364211d3d5279bb18e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pb9-9446000000-88b3a1e8347409cbd90f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0009000000-93e71df8c273f316291e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000t-0009000000-c213a2049e0227593613 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0159-3009000000-46a0e83c43d9afaf6604 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0009000000-dc23ba87137530d83f68 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0009000000-dc23ba87137530d83f68 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-0009000000-43c77f5a7a78cef88d7d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0014900000-1eb6d2fb34f5efea9b0e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0k9f-5095200000-9335d6525175b1a5fead | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9742000000-8137e269062cdeb912b8 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0034328 |
|---|
| FooDB ID | FDB012686 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131751546 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|