| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:22:08 UTC |
|---|
| Update Date | 2016-11-09 01:18:39 UTC |
|---|
| Accession Number | CHEM028309 |
|---|
| Identification |
|---|
| Common Name | Thiolutin |
|---|
| Class | Small Molecule |
|---|
| Description | A dithiolopyrrolone antibiotic that is 4,5-dihydro[1,2]dithiolo[4,3-b]pyrrole in which the hydrogens at positions 4,5 and 6 have been replaced by methyl, oxo and acetamido groups, respectively. It is a potent inhibitor of RNA polymerases, inhibits the angiogenesis of human umbilical vein endothelial cells, and also inhibits JAMM metalloproteases. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
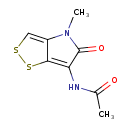
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 6-Acetamido-4-methyl-1,2-dithiolo[4,3-b]pyrrol-5(4H)-one | ChEBI | | Acetopyrrothin | ChEBI | | Acetopyrrothine | ChEBI | | N-(4,5-Dihydro-4-methyl-5-oxo-1,2-dithiolo[4,3-b]pyrrol-6-yl)acetamide | ChEBI | | N-(4-Methyl-5-oxodithiolo[4,3-b]pyrrol-6-yl)acetamide | ChEBI | | Farcinicine | HMDB | | N-Acetylpyrrothine | HMDB | | N-{4-methyl-5-oxo-4H,5H-[1,2]dithiolo[4,3-b]pyrrol-6-yl}ethanimidate | Generator | | Thiolutin | MeSH |
|
|---|
| Chemical Formula | C8H8N2O2S2 |
|---|
| Average Molecular Mass | 228.291 g/mol |
|---|
| Monoisotopic Mass | 228.003 g/mol |
|---|
| CAS Registry Number | 87-11-6 |
|---|
| IUPAC Name | N-{4-methyl-5-oxo-4H,5H-[1,2]dithiolo[4,3-b]pyrrol-6-yl}acetamide |
|---|
| Traditional Name | thiolutin |
|---|
| SMILES | CN1C(=O)C(NC(C)=O)=C2SSC=C12 |
|---|
| InChI Identifier | InChI=1S/C8H8N2O2S2/c1-4(11)9-6-7-5(3-13-14-7)10(2)8(6)12/h3H,1-2H3,(H,9,11) |
|---|
| InChI Key | MHMRAFONCSQAIA-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as n-acetylarylamines. These are acetamides where one or more amide hydrogens is substituted by an aryl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic nitrogen compounds |
|---|
| Class | Organonitrogen compounds |
|---|
| Sub Class | N-arylamides |
|---|
| Direct Parent | N-acetylarylamines |
|---|
| Alternative Parents | |
|---|
| Substituents | - N-acetylarylamine
- N-methylpyrrole
- Substituted pyrrole
- Pyrrole
- 1,2-dithiole
- Dithiole
- Acetamide
- Heteroaromatic compound
- Carboxamide group
- Secondary carboxylic acid amide
- Lactam
- Organoheterocyclic compound
- Carboxylic acid derivative
- Azacycle
- Carbonyl group
- Organopnictogen compound
- Organooxygen compound
- Organic oxygen compound
- Hydrocarbon derivative
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-054o-3910000000-519ee727a019d3a93d69 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-7590000000-85817f7eb87b0115bd93 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004r-0590000000-515631cb29113ba3b962 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052s-9200000000-f7ff9dbd9f3a2082f78f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0890000000-9e6266701a4ec9c61060 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-003r-1970000000-03b1d843e3d93fbf40bc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0zgi-2900000000-2d15a380d25bc120cdf3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002r-0980000000-5f26834e99b886e3f03b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-002r-0960000000-7693ffa3bb0a88d601d7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0159-2900000000-a10fda5b87d577f08ad2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0090000000-95faf19c58c835a83e00 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0059-0590000000-0ab84e20d5f22fd4f7af | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-1920000000-229b3b69617b7784c357 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0034228 |
|---|
| FooDB ID | FDB012539 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00054277 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | CPD-17934 |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Thiolutin |
|---|
| Chemspider ID | 6608 |
|---|
| ChEBI ID | 156450 |
|---|
| PubChem Compound ID | 6870 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. https://www.ncbi.nlm.nih.gov/pubmed/?term=11433393 | | 2. https://www.ncbi.nlm.nih.gov/pubmed/?term=17036064 | | 3. https://www.ncbi.nlm.nih.gov/pubmed/?term=17914747 | | 4. https://www.ncbi.nlm.nih.gov/pubmed/?term=18720982 | | 5. https://www.ncbi.nlm.nih.gov/pubmed/?term=19579057 | | 6. https://www.ncbi.nlm.nih.gov/pubmed/?term=2262169 | | 7. https://www.ncbi.nlm.nih.gov/pubmed/?term=24723205 | | 8. https://www.ncbi.nlm.nih.gov/pubmed/?term=25353334 | | 9. https://www.ncbi.nlm.nih.gov/pubmed/?term=28459440 | | 10. https://www.ncbi.nlm.nih.gov/pubmed/?term=31601735 | | 11. https://www.ncbi.nlm.nih.gov/pubmed/?term=32049520 | | 12. https://www.ncbi.nlm.nih.gov/pubmed/?term=4583213 | | 13. https://www.ncbi.nlm.nih.gov/pubmed/?term=4597739 | | 14. https://www.ncbi.nlm.nih.gov/pubmed/?term=4604615 | | 15. https://www.ncbi.nlm.nih.gov/pubmed/?term=6758684 | | 16. https://www.ncbi.nlm.nih.gov/pubmed/?term=775314 | | 17. https://www.ncbi.nlm.nih.gov/pubmed/?term=793624 | | 18. Dai S, Jia Y, Wu SL, Isenberg JS, Ridnour LA, Bandle RW, Wink DA, Roberts DD, Karger BL: Comprehensive characterization of heat shock protein 27 phosphorylation in human endothelial cells stimulated by the microbial dithiole thiolutin. J Proteome Res. 2008 Oct;7(10):4384-95. doi: 10.1021/pr800376w. Epub 2008 Aug 23. | | 19. Yannai, Shmuel. (2004) Dictionary of food compounds with CD-ROM: Additives, flavors, and ingredients. Boca Raton: Chapman & Hall/CRC. |
|
|---|