| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:20:20 UTC |
|---|
| Update Date | 2016-11-09 01:18:39 UTC |
|---|
| Accession Number | CHEM028268 |
|---|
| Identification |
|---|
| Common Name | Protohypericin |
|---|
| Class | Small Molecule |
|---|
| Description | Isolated from Hypericum perforatum (St. John's Wort). Protohypericin is found in tea, alcoholic beverages, and herbs and spices. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
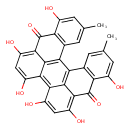
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Protohypericin | MeSH |
|
|---|
| Chemical Formula | C30H18O8 |
|---|
| Average Molecular Mass | 506.459 g/mol |
|---|
| Monoisotopic Mass | 506.100 g/mol |
|---|
| CAS Registry Number | 548-03-8 |
|---|
| IUPAC Name | 7,11,13,16,18,22-hexahydroxy-5,24-dimethylheptacyclo[13.11.1.1²,¹⁰.0³,⁸.0¹⁹,²⁷.0²¹,²⁶.0¹⁴,²⁸]octacosa-1(27),2(28),3(8),4,6,10,12,14,16,18,21(26),22,24-tridecaene-9,20-dione |
|---|
| Traditional Name | 7,11,13,16,18,22-hexahydroxy-5,24-dimethylheptacyclo[13.11.1.1²,¹⁰.0³,⁸.0¹⁹,²⁷.0²¹,²⁶.0¹⁴,²⁸]octacosa-1(27),2(28),3(8),4,6,10,12,14,16,18,21(26),22,24-tridecaene-9,20-dione |
|---|
| SMILES | CC1=CC2=C(C(O)=C1)C(=O)C1=C(O)C=C(O)C3=C4C(O)=CC(O)=C5C(=O)C6=C(C=C(C)C=C6O)C(C2=C13)=C45 |
|---|
| InChI Identifier | InChI=1S/C30H18O8/c1-9-3-11-19(13(31)5-9)29(37)25-17(35)7-15(33)23-24-16(34)8-18(36)26-28(24)22(21(11)27(23)25)12-4-10(2)6-14(32)20(12)30(26)38/h3-8,31-36H,1-2H3 |
|---|
| InChI Key | YLILOANQCQKPOD-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as perylenequinones. These are heterocyclic compounds characterized by two 8-hydroxy-1,4-dihydronaphthalen-1-one moieties joined together one or two CC-bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Perylenequinones |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Perylenequinones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Perylenequinone
- Phenanthrol
- Phenanthrene
- Anthracene
- 1-naphthol
- 2-naphthol
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Vinylogous acid
- Polyol
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-056r-0100930000-12336cd4cee0283cdc28 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0079-5100059000-a013c10cef25f6b4ee31 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0000290000-b285ed6a5bbc67d2561e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0000590000-b687985249b486ac4934 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0079-1000900000-59633c5f097a37bebecb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0000090000-c055ae27121e2e0cd65a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0000190000-70652925180413f71f54 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-2000910000-c6565155993cc2f93715 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0000090000-6ebfacadbc229ed0c1c6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0000090000-6ebfacadbc229ed0c1c6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-0000290000-e1e9d557e94689a83a64 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0000090000-cd3514b49df87a50d72d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0000090000-cd3514b49df87a50d72d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004r-0000900000-7ddadd933a23e5889a23 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0034180 |
|---|
| FooDB ID | FDB012472 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00034650 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4590166 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 5489488 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|