| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:19:24 UTC |
|---|
| Update Date | 2016-11-09 01:18:39 UTC |
|---|
| Accession Number | CHEM028251 |
|---|
| Identification |
|---|
| Common Name | (-)-cis-Rotenolone |
|---|
| Class | Small Molecule |
|---|
| Description | (-)-cis-Rotenolone is found in jicama. (-)-cis-Rotenolone is isolated from Pachyrrhizus erosus (yam bean). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
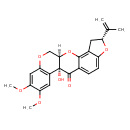
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (-)-cis-12a-Hydroxyrotenone | HMDB | | 1,2,12,12a-tetrahydro-6a-Hydroxy-8,9-dimethoxy-2-(1-methylethenyl)-(2R,6ar,12ar)-[1]benzopyrano[3,4-b]furo[2,3-H][1]benzopyran-6(6ah)-one | HMDB | | 12-Hydroxyrotenone | HMDB | | 12alpha-Hydroxyrotenone | HMDB | | 6a-beta,12a-beta-Rotenolone | HMDB | | 6Ab,12ab-rotenolone | HMDB | | Rotenalone | HMDB | | Rotenolon I | HMDB | | Rotenolone | HMDB | | Rotenolone I | HMDB | | 6 alpha(beta),12 alpha(beta)-Rotenolone | MeSH | | Rotenolone, (2R-(2alpha,6aalpha,12aalpha))-isomer | MeSH | | Rotenolone, (2R-(2alpha,6abeta,12aalpha))-isomer | MeSH | | Rotenolone, (2R-(2alpha,6aalpha,12abeta))-isomer | MeSH |
|
|---|
| Chemical Formula | C23H22O7 |
|---|
| Average Molecular Mass | 410.417 g/mol |
|---|
| Monoisotopic Mass | 410.137 g/mol |
|---|
| CAS Registry Number | 509-96-6 |
|---|
| IUPAC Name | (1R,6R,13R)-13-hydroxy-16,17-dimethoxy-6-(prop-1-en-2-yl)-2,7,20-trioxapentacyclo[11.8.0.0³,¹¹.0⁴,⁸.0¹⁴,¹⁹]henicosa-3,8,10,14(19),15,17-hexaen-12-one |
|---|
| Traditional Name | rotenolone |
|---|
| SMILES | [H][C@@]12COC3=C(C=C(OC)C(OC)=C3)[C@]1(O)C(=O)C1=CC=C3O[C@H](CC3=C1O2)C(C)=C |
|---|
| InChI Identifier | InChI=1S/C23H22O7/c1-11(2)16-7-13-15(29-16)6-5-12-21(13)30-20-10-28-17-9-19(27-4)18(26-3)8-14(17)23(20,25)22(12)24/h5-6,8-9,16,20,25H,1,7,10H2,2-4H3/t16-,20-,23-/m1/s1 |
|---|
| InChI Key | JFVKWCYZKMUTLH-AYPBNUJASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as rotenones. These are rotenoids with a structure based on a 6a,12a-dihydrochromeno[3,4-b]chromen-12(6H)-one skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Isoflavonoids |
|---|
| Sub Class | Rotenoids |
|---|
| Direct Parent | Rotenones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Rotenone or derivatives
- 8-prenylated isoflavanone
- Isoflavanone
- Isoflavan
- Chromone
- 1-benzopyran
- Chromane
- Benzopyran
- Coumaran
- Aryl alkyl ketone
- Aryl ketone
- Anisole
- Alkyl aryl ether
- Benzenoid
- Tertiary alcohol
- Ketone
- Ether
- Oxacycle
- Organoheterocyclic compound
- Organic oxygen compound
- Alcohol
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0udj-2898000000-a911918046b993e78796 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0v4i-9420700000-d42a2e5be951b642755e | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-1223900000-9a1f17cd964abaa81aa8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0w29-2394300000-b8d33d58c295207f77a3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-2930000000-7ebdad6df07b04059cf4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0011900000-068963a903131042e2de | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0059600000-b085e22dff915ed213c1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udr-1933000000-acb18ae95eb8c0f59fb9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0000900000-13ca06aa040c9821ae69 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0325900000-33ff0fbbe9523120bd61 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-1869100000-793032f9edca87024cfd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0000900000-cc474c0f71581fe9b76f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0115900000-866e3635b32607356e9c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0zi0-3395000000-409148100e6d88cb50cd | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0034145 |
|---|
| FooDB ID | FDB012420 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00002537 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 61491 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 68184 |
|---|
| Kegg Compound ID | C10464 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|