| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:18:33 UTC |
|---|
| Update Date | 2016-11-09 01:18:38 UTC |
|---|
| Accession Number | CHEM028229 |
|---|
| Identification |
|---|
| Common Name | 6alpha-Hydroxymedicarpin |
|---|
| Class | Small Molecule |
|---|
| Description | Constituent of rhubarb (Rheum rhaponticum) root. (E)-4'-Methylresveratrol 3-glucoside is found in green vegetables. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
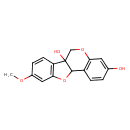
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3,6a-Dihydroxy-9-methoxypterocarpan | HMDB | | 6a-Hydroxymedicarpin | HMDB, Generator | | 6Α-hydroxymedicarpin | Generator |
|
|---|
| Chemical Formula | C16H14O5 |
|---|
| Average Molecular Mass | 286.279 g/mol |
|---|
| Monoisotopic Mass | 286.084 g/mol |
|---|
| CAS Registry Number | 61135-92-0 |
|---|
| IUPAC Name | 14-methoxy-8,17-dioxatetracyclo[8.7.0.0²,⁷.0¹¹,¹⁶]heptadeca-2(7),3,5,11(16),12,14-hexaene-5,10-diol |
|---|
| Traditional Name | 14-methoxy-8,17-dioxatetracyclo[8.7.0.0²,⁷.0¹¹,¹⁶]heptadeca-2(7),3,5,11(16),12,14-hexaene-5,10-diol |
|---|
| SMILES | COC1=CC2=C(C=C1)C1(O)COC3=C(C=CC(O)=C3)C1O2 |
|---|
| InChI Identifier | InChI=1S/C16H14O5/c1-19-10-3-5-12-14(7-10)21-15-11-4-2-9(17)6-13(11)20-8-16(12,15)18/h2-7,15,17-18H,8H2,1H3 |
|---|
| InChI Key | SXKBOSYKWYQHMV-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as stilbene glycosides. Stilbene glycosides are compounds structurally characterized by the presence of a carbohydrate moiety glycosidically linked to the stilbene skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Stilbenes |
|---|
| Sub Class | Stilbene glycosides |
|---|
| Direct Parent | Stilbene glycosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Stilbene glycoside
- Phenolic glycoside
- Hexose monosaccharide
- Glycosyl compound
- O-glycosyl compound
- Anisole
- Phenoxy compound
- Phenol ether
- Styrene
- Methoxybenzene
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Phenol
- Benzenoid
- Monocyclic benzene moiety
- Monosaccharide
- Oxane
- Secondary alcohol
- Acetal
- Ether
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Organooxygen compound
- Organic oxygen compound
- Alcohol
- Hydrocarbon derivative
- Primary alcohol
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0600-1690000000-346bdcac066b6f381678 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-06fu-6629800000-b237ef9c110b76f2ec1f | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0190000000-3d79e967ab74798677ce | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0090000000-311f4c987f635cd9a076 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pec-9510000000-5faf9627b9b4723bb4c1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-5f41d188549e4f62e707 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0090000000-1b1ec91bb3a5d817972c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0670-3290000000-1831012c4879e89f261c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-14912e1703be5edd027d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0090000000-c93a26febaac60ba7a41 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0uxr-0090000000-acdcb7fb8a3e27b00b6b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0090000000-b239d41c6032c85540b2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0290000000-dec61ecd14cc98e8a255 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00y0-1940000000-4702bd8b4c2523bbaa73 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0034117 |
|---|
| FooDB ID | FDB012385 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131751528 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|