| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:17:55 UTC |
|---|
| Update Date | 2016-11-09 01:18:38 UTC |
|---|
| Accession Number | CHEM028214 |
|---|
| Identification |
|---|
| Common Name | (2E,4E)-2,7-Dimethyl-2,4-octadienedioic acid |
|---|
| Class | Small Molecule |
|---|
| Description | (2E,4E)-2,7-Dimethyl-2,4-octadienedioic acid is found in garden tomato. (2E,4E)-2,7-Dimethyl-2,4-octadienedioic acid is produced by a mutant tomato variety 'flacca' deficient in abscisic acid. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (2E,4E)-2,7-Dimethyl-2,4-octadienedioate | Generator | | (2E,4E)-2,7-Dimethylocta-2,4-dienedioate | HMDB |
|
|---|
| Chemical Formula | C10H14O4 |
|---|
| Average Molecular Mass | 198.216 g/mol |
|---|
| Monoisotopic Mass | 198.089 g/mol |
|---|
| CAS Registry Number | 110107-15-8 |
|---|
| IUPAC Name | (2E,4E)-2,7-dimethylocta-2,4-dienedioic acid |
|---|
| Traditional Name | (2E,4E)-2,7-dimethylocta-2,4-dienedioic acid |
|---|
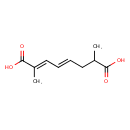
| SMILES | CC(C\C=C\C=C(/C)C(O)=O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C10H14O4/c1-7(9(11)12)5-3-4-6-8(2)10(13)14/h3-5,8H,6H2,1-2H3,(H,11,12)(H,13,14)/b4-3+,7-5+ |
|---|
| InChI Key | ZBXYMKUZCWCGPC-BDWKERMESA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as medium-chain fatty acids. These are fatty acids with an aliphatic tail that contains between 4 and 12 carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acids and conjugates |
|---|
| Direct Parent | Medium-chain fatty acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Medium-chain fatty acid
- Methyl-branched fatty acid
- Branched fatty acid
- Unsaturated fatty acid
- Dicarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0fi3-6900000000-12e7aff180eb1b681d0b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00fr-9221000000-c91ee355d3a349f31559 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0900000000-25a79c8e005ceca5cfd5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fc1-3900000000-ad0c0ae1a8b982efac48 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9700000000-d309cc31171fd262fefe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-db0c1e584ddfe751970e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0f6t-0900000000-ce20476435b9c13fe484 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0abc-9700000000-252575cdae89708d505a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-894d391d8374fa52e9a1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-1900000000-be85634741f23bab0b61 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0059-9200000000-fe7abad923fa8624e84d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0032-1900000000-f2ca447f4c25010b3a9b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00or-9000000000-03c17603534dbee984d9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-016r-9200000000-011f7880e95d942d8634 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0034099 |
|---|
| FooDB ID | FDB012363 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 9129733 |
|---|
| ChEBI ID | 173995 |
|---|
| PubChem Compound ID | 10954516 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|