| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:17:46 UTC |
|---|
| Update Date | 2016-11-09 01:18:38 UTC |
|---|
| Accession Number | CHEM028210 |
|---|
| Identification |
|---|
| Common Name | Gambogic acid |
|---|
| Class | Small Molecule |
|---|
| Description | Isolated from Gamboge resin (exudate of Garcinia morella). Gambogic acid is found in herbs and spices and fruits. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
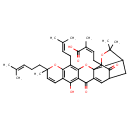
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Gambogate | Generator | | a-Gambogic acid | HMDB | | b-Guttiferin | HMDB | | b-Guttilactone | HMDB | | Guttatic acid | HMDB | | Guttic acid | HMDB | | Gamboge acid | HMDB | | Gambogoic acid | HMDB | | (2Z)-4-[12-Hydroxy-8,21,21-trimethyl-5-(3-methylbut-2-en-1-yl)-8-(4-methylpent-3-en-1-yl)-14,18-dioxo-3,7,20-trioxahexacyclo[15.4.1.0²,¹⁵.0²,¹⁹.0⁴,¹³.0⁶,¹¹]docosa-4(13),5,9,11,15-pentaen-19-yl]-2-methylbut-2-enoate | Generator | | Gambogic acid | MeSH |
|
|---|
| Chemical Formula | C38H44O8 |
|---|
| Average Molecular Mass | 628.751 g/mol |
|---|
| Monoisotopic Mass | 628.304 g/mol |
|---|
| CAS Registry Number | 2752-65-0 |
|---|
| IUPAC Name | (2Z)-4-[12-hydroxy-8,21,21-trimethyl-5-(3-methylbut-2-en-1-yl)-8-(4-methylpent-3-en-1-yl)-14,18-dioxo-3,7,20-trioxahexacyclo[15.4.1.0²,¹⁵.0²,¹⁹.0⁴,¹³.0⁶,¹¹]docosa-4,6(11),9,12,15-pentaen-19-yl]-2-methylbut-2-enoic acid |
|---|
| Traditional Name | (2Z)-4-[12-hydroxy-8,21,21-trimethyl-5-(3-methylbut-2-en-1-yl)-8-(4-methylpent-3-en-1-yl)-14,18-dioxo-3,7,20-trioxahexacyclo[15.4.1.0²,¹⁵.0²,¹⁹.0⁴,¹³.0⁶,¹¹]docosa-4,6(11),9,12,15-pentaen-19-yl]-2-methylbut-2-enoic acid |
|---|
| SMILES | CC(C)=CCCC1(C)OC2=C(C=C1)C(O)=C1C(=O)C3=CC4CC5C(C)(C)OC(C\C=C(\C)C(O)=O)(C4=O)C35OC1=C2CC=C(C)C |
|---|
| InChI Identifier | InChI=1S/C38H44O8/c1-20(2)10-9-15-36(8)16-14-24-29(39)28-30(40)26-18-23-19-27-35(6,7)46-37(33(23)41,17-13-22(5)34(42)43)38(26,27)45-32(28)25(31(24)44-36)12-11-21(3)4/h10-11,13-14,16,18,23,27,39H,9,12,15,17,19H2,1-8H3,(H,42,43)/b22-13- |
|---|
| InChI Key | GEZHEQNLKAOMCA-XKZIYDEJSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyranoxanthones. These are organic aromatic compounds containing a pyran or a hydrogenated derivative fused to a xanthone ring system. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzopyrans |
|---|
| Sub Class | 1-benzopyrans |
|---|
| Direct Parent | Pyranoxanthones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyranoxanthone
- Pyranochromene
- Chromone
- Aromatic monoterpenoid
- Monoterpenoid
- Aryl ketone
- Alkyl aryl ether
- Cyclohexenone
- Oxepane
- Benzenoid
- Vinylogous acid
- Tetrahydrofuran
- Ketone
- Monocarboxylic acid or derivatives
- Oxacycle
- Ether
- Dialkyl ether
- Carboxylic acid
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Organic oxygen compound
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-03di-9000057000-19abde6d758101a01ef7 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("Gambogic acid,1TMS,#1" TMS) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-1001039000-9c55dff29165d33866d8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-057i-1014095000-77a8e0d8dca00f9672ef | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ab9-2794313000-d45dab05ad38be7aee92 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0000009000-312b00b9e28384b5d53e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0000059000-d354ba3ab7fc6d3f1462 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-02t9-2100391000-dcdb58e0da6ce0fbbe96 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01t9-0000096000-e56a0ec531207df34ab6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0o6r-3001591000-411cf0af71ea4c9389ec | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-016r-7153941000-92579129327c5702bfb8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0000079000-d5501837c889acaa5034 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004r-0000093000-23942b0217f9c03ee878 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00kg-2000951000-5ee22b87ea31bfe4e09e | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0034095 |
|---|
| FooDB ID | FDB012358 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00002950 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Gambogic acid |
|---|
| Chemspider ID | 4510165 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 5353639 |
|---|
| Kegg Compound ID | C10062 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|