| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:16:40 UTC |
|---|
| Update Date | 2016-11-09 01:18:38 UTC |
|---|
| Accession Number | CHEM028186 |
|---|
| Identification |
|---|
| Common Name | Gentianose |
|---|
| Class | Small Molecule |
|---|
| Description | Raffinose is a trisaccharide composed of galactose, glucose, and fructose. It can be found in beans, cabbage, brussels sprouts, broccoli, asparagus, other vegetables, and whole grains. Raffinose can be hydrolyzed to D-galactose and sucrose by the enzyme α-galactosidase (α-GAL), an enzyme not found in the human digestive tract. α-GAL also hydrolyzes other α-galactosides such as stachyose, verbascose, and galactinol, if present. The enzyme does not cleave β-linked galactose, as in lactose. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
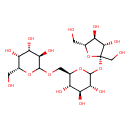
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 6g-alpha-D-Galactosylsucrose | HMDB | | 6g-alpha-delta-Galactosylsucrose | HMDB | | D-(+)-Raffinose | HMDB | | D-Raffinose | HMDB | | delta-(+)-Raffinose | HMDB | | delta-Raffinose | HMDB | | Gossypose | HMDB | | Melitose | HMDB | | Melitriose | HMDB | | Raffinose hydrate | HMDB |
|
|---|
| Chemical Formula | C18H32O16 |
|---|
| Average Molecular Mass | 504.437 g/mol |
|---|
| Monoisotopic Mass | 504.169 g/mol |
|---|
| CAS Registry Number | 25954-44-3 |
|---|
| IUPAC Name | (3R,4S,5R,6R)-2-{[(2R,3S,4S,5R)-6-{[(2S,3S,4S,5R)-3,4-dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl]oxy}-3,4,5-trihydroxyoxan-2-yl]methoxy}-6-(hydroxymethyl)oxane-3,4,5-triol |
|---|
| Traditional Name | (3R,4S,5R,6R)-2-{[(2R,3S,4S,5R)-6-{[(2S,3S,4S,5R)-3,4-dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl]oxy}-3,4,5-trihydroxyoxan-2-yl]methoxy}-6-(hydroxymethyl)oxane-3,4,5-triol |
|---|
| SMILES | OCC1OC(CO)(OC2OC(COC3OC(CO)C(O)C(O)C3O)C(O)C(O)C2O)C(O)C1O |
|---|
| InChI Identifier | InChI=1S/C18H32O16/c19-1-5-8(22)11(25)13(27)16(31-5)30-3-7-9(23)12(26)14(28)17(32-7)34-18(4-21)15(29)10(24)6(2-20)33-18/h5-17,19-29H,1-4H2 |
|---|
| InChI Key | MUPFEKGTMRGPLJ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as oligosaccharides. These are carbohydrates made up of 3 to 10 monosaccharide units linked to each other through glycosidic bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Oligosaccharides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Oligosaccharide
- C-glycosyl compound
- Glycosyl compound
- O-glycosyl compound
- Ketal
- Oxane
- Tetrahydrofuran
- Secondary alcohol
- Acetal
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Alcohol
- Primary alcohol
- Hydrocarbon derivative
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0079-5312900000-8c1da6b96f865046dbc2 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0l1i-7432149000-e8883b57e0531dff1cc0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01q9-0901000000-64f2e5bde9015fd5ad69 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03gi-0906000000-fc81b4e448a153802db5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03dl-9800000000-c8990312d1658c9255a1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-2900000000-b81d8d3ee7919e19296a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03fr-0900000000-319ebbeb5b37f8ed7f2d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-002e-6900000000-6b955be165071ff0a635 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Raffinose |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 10542 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | ECMDB03213 |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|