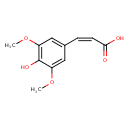| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:16:26 UTC |
|---|
| Update Date | 2016-11-09 01:18:38 UTC |
|---|
| Accession Number | CHEM028181 |
|---|
| Identification |
|---|
| Common Name | cis-Sinapic acid |
|---|
| Class | Small Molecule |
|---|
| Description | A 3-(4-hydroxy-3,5-dimethoxyphenyl)prop-2-enoic acid in which the double bond has cis-configuration. It has been isolated from the shoots of alfalfa. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (Z)-4-Hydroxy-3,5-dimethoxycinnamic acid | ChEBI | | cis-4-Hydroxy-3,5-dimethoxycinnamic acid | ChEBI | | (Z)-4-Hydroxy-3,5-dimethoxycinnamate | Generator | | cis-4-Hydroxy-3,5-dimethoxycinnamate | Generator | | cis-Sinapate | Generator | | trans-Sinapinic acid | MeSH, HMDB | | 3,5-Dimethoxy-4-hydroxycinnamic acid | MeSH, HMDB | | (e)-Sinapic acid | MeSH, HMDB | | Sinapic acid | MeSH, HMDB | | Sinapinic acid | MeSH, HMDB | | Synapitic acid | MeSH, HMDB | | 4-Hydroxy-3,5-dimethoxycinnamic acid | MeSH, HMDB | | trans-Sinapic acid | MeSH, HMDB | | (2Z)-3-(4-Hydroxy-3,5-dimethoxyphenyl)-2-propenoic acid | HMDB | | (Z)-3,5-Dimethoxy-4-hydroxycinnamic acid | HMDB | | (Z)-3-(4-Hydroxy-3,5-dimethoxyphenyl)acrylic acid | HMDB | | (Z)-Sinapic acid | HMDB | | 3,5-Dimethoxy-4-hydroxy-cis-cinnamic acid | HMDB | | 3-(4-Hydroxy-3,5-dimethoxyphenyl)-2-propenoic acid | HMDB | | Z-Sinapinic acid | HMDB | | cis-Sinapic acid | HMDB | | cis-Sinapinic acid | HMDB |
|
|---|
| Chemical Formula | C11H12O5 |
|---|
| Average Molecular Mass | 224.210 g/mol |
|---|
| Monoisotopic Mass | 224.068 g/mol |
|---|
| CAS Registry Number | 7361-90-2 |
|---|
| IUPAC Name | (2Z)-3-(4-hydroxy-3,5-dimethoxyphenyl)prop-2-enoic acid |
|---|
| Traditional Name | (2Z)-3-(4-hydroxy-3,5-dimethoxyphenyl)prop-2-enoic acid |
|---|
| SMILES | COC1=CC(\C=C/C(O)=O)=CC(OC)=C1O |
|---|
| InChI Identifier | InChI=1S/C11H12O5/c1-15-8-5-7(3-4-10(12)13)6-9(16-2)11(8)14/h3-6,14H,1-2H3,(H,12,13)/b4-3- |
|---|
| InChI Key | PCMORTLOPMLEFB-ARJAWSKDSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as hydroxycinnamic acids. Hydroxycinnamic acids are compounds containing an cinnamic acid where the benzene ring is hydroxylated. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Cinnamic acids and derivatives |
|---|
| Sub Class | Hydroxycinnamic acids and derivatives |
|---|
| Direct Parent | Hydroxycinnamic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cinnamic acid
- Coumaric acid or derivatives
- Hydroxycinnamic acid
- M-dimethoxybenzene
- Dimethoxybenzene
- Methoxyphenol
- Phenoxy compound
- Anisole
- Methoxybenzene
- Styrene
- Phenol ether
- Alkyl aryl ether
- Phenol
- Monocyclic benzene moiety
- Benzenoid
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Ether
- Organic oxygen compound
- Carbonyl group
- Organic oxide
- Organooxygen compound
- Hydrocarbon derivative
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | - 3-(4-hydroxy-3,5-dimethoxyphenyl)prop-2-enoic acid (CHEBI:76350 )
|
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (2 TMS) | splash10-00kr-3659000000-141953a5d5d848f1aa51 | Spectrum | | GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-00kr-3659000000-141953a5d5d848f1aa51 | Spectrum | | GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-000i-2958000000-16d208a44d4442af41c5 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05di-1960000000-e20c10aa0bcc765f8c28 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0umi-8196000000-afceb35e7a5f28ec225c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-056r-0190000000-6e81a94093485194a43f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-1960000000-18717b9e874f0901ea68 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-06vj-2900000000-254ee08e710708eb6545 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0190000000-89ae701e731ef1f24fd7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0890000000-74c0bdbe7769b328017a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-1910000000-69bbc48ce24397a342a2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a6r-0290000000-9de305bfb1242c5c3e10 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-056r-0980000000-368fb5936979c9a6e99d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0aor-3900000000-395e080ff0323f7c60bc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0090000000-cfca81bd3cf2894d0d6b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0900000000-e363c6280db16d0c6ea7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00dl-8930000000-4fe3d11040b797e9d211 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0034069 |
|---|
| FooDB ID | FDB012318 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 1266059 |
|---|
| ChEBI ID | 76350 |
|---|
| PubChem Compound ID | 1549091 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|