| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:15:20 UTC |
|---|
| Update Date | 2016-11-09 01:18:37 UTC |
|---|
| Accession Number | CHEM028153 |
|---|
| Identification |
|---|
| Common Name | Tricetanidin |
|---|
| Class | Small Molecule |
|---|
| Description | Tricetanidin is found in tea. Tricetanidin is isolated from black tea (Thea sinensis). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
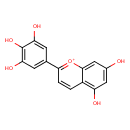
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 5,7-Dihydroxy-2-(3,4,5-trihydroxyphenyl)-1-benzopyrylium(1+), 9ci | HMDB | | Tricetinidin | HMDB | | Tricetinidin chloride | HMDB |
|
|---|
| Chemical Formula | C15H11O6 |
|---|
| Average Molecular Mass | 287.244 g/mol |
|---|
| Monoisotopic Mass | 287.056 g/mol |
|---|
| CAS Registry Number | 65618-21-5 |
|---|
| IUPAC Name | 5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-1λ⁴-chromen-1-ylium |
|---|
| Traditional Name | tricetinidin |
|---|
| SMILES | OC1=CC(O)=C2C=CC(=[O+]C2=C1)C1=CC(O)=C(O)C(O)=C1 |
|---|
| InChI Identifier | InChI=1S/C15H10O6/c16-8-5-10(17)9-1-2-13(21-14(9)6-8)7-3-11(18)15(20)12(19)4-7/h1-6H,(H4-,16,17,18,19,20)/p+1 |
|---|
| InChI Key | CMPNIWQMRYYTMK-UHFFFAOYSA-O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 7-hydroxyflavonoids. These are flavonoids that bear one hydroxyl group at the C-7 position of the flavonoid skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Flavonoids |
|---|
| Sub Class | Hydroxyflavonoids |
|---|
| Direct Parent | 7-hydroxyflavonoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - 3'-hydroxyflavonoid
- 4'-hydroxyflavonoid
- 5-hydroxyflavonoid
- 7-hydroxyflavonoid
- Anthocyanidin
- 1-benzopyran
- Benzopyran
- Pyrogallol derivative
- Benzenetriol
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Benzenoid
- Monocyclic benzene moiety
- Heteroaromatic compound
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Hydrocarbon derivative
- Organic oxygen compound
- Organooxygen compound
- Organic cation
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05n0-0690000000-f3ad6bda47bb8567d643 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (5 TMS) - 70eV, Positive | splash10-05ai-3011195000-51fcc65ca55beb4776fd | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0090000000-2828ce6d57a30f37ae48 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0090000000-af7716dcbc2f35dbcace | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00tr-0590000000-7a62d2df8f85ddfc2bec | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-acf2030f20759837048a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0090000000-9bd532c7a371a01325c5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-5190000000-49c2fa13e54a456de9fb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0090000000-d06e4a8548a2a82e8dc0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0090000000-8bc5dd6fb897c491cb64 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00kr-0790000000-ee4ecec9faa0c6686bd7 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0034043 |
|---|
| FooDB ID | FDB012285 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00006612 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 9374719 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 11199650 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|