| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:14:52 UTC |
|---|
| Update Date | 2016-11-09 01:18:37 UTC |
|---|
| Accession Number | CHEM028142 |
|---|
| Identification |
|---|
| Common Name | 6-(2-Carboxyethyl)-7-hydroxy-2,2-dimethyl-4-chromanone glucoside |
|---|
| Class | Small Molecule |
|---|
| Description | 6-(2-Carboxyethyl)-7-hydroxy-2,2-dimethyl-4-chromanone glucoside is found in herbs and spices. 6-(2-Carboxyethyl)-7-hydroxy-2,2-dimethyl-4-chromanone glucoside is a constituent of fennel, Foeniculum vulgare. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
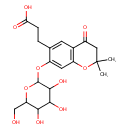
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3-(2,2-Dimethyl-4-oxo-7-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-3,4-dihydro-2H-1-benzopyran-6-yl)propanoate | HMDB |
|
|---|
| Chemical Formula | C20H26O10 |
|---|
| Average Molecular Mass | 426.414 g/mol |
|---|
| Monoisotopic Mass | 426.153 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 3-(2,2-dimethyl-4-oxo-7-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-3,4-dihydro-2H-1-benzopyran-6-yl)propanoic acid |
|---|
| Traditional Name | 3-(2,2-dimethyl-4-oxo-7-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-3H-1-benzopyran-6-yl)propanoic acid |
|---|
| SMILES | CC1(C)CC(=O)C2=C(O1)C=C(OC1OC(CO)C(O)C(O)C1O)C(CCC(O)=O)=C2 |
|---|
| InChI Identifier | InChI=1S/C20H26O10/c1-20(2)7-11(22)10-5-9(3-4-15(23)24)12(6-13(10)30-20)28-19-18(27)17(26)16(25)14(8-21)29-19/h5-6,14,16-19,21,25-27H,3-4,7-8H2,1-2H3,(H,23,24) |
|---|
| InChI Key | DSNULGSRJZPYOC-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenolic glycosides. These are organic compounds containing a phenolic structure attached to a glycosyl moiety. Some examples of phenolic structures include lignans, and flavonoids. Among the sugar units found in natural glycosides are D-glucose, L-Fructose, and L rhamnose. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Phenolic glycosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenolic glycoside
- Hexose monosaccharide
- 2,2-dimethyl-1-benzopyran
- Chromone
- O-glycosyl compound
- Chromane
- Benzopyran
- 1-benzopyran
- Aryl alkyl ketone
- Aryl ketone
- Alkyl aryl ether
- Benzenoid
- Monosaccharide
- Oxane
- Secondary alcohol
- Ketone
- Monocarboxylic acid or derivatives
- Acetal
- Ether
- Organoheterocyclic compound
- Carboxylic acid
- Carboxylic acid derivative
- Oxacycle
- Polyol
- Alcohol
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Primary alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-9318300000-1b9a974bb481cb18eac1 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-004i-4332069000-cdcd622f5d95cd8bff4d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0691-0194600000-187da7ac80d83a362000 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014j-0190000000-abf2cc193da2443ff619 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-066r-4490000000-311139fbc764abcd6809 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01t9-1262900000-865d1291b7836dc80e71 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-1191100000-c7ad13b4dff8521f58f9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-08fs-7090000000-3318133683636ed2f87b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-05r0-0051900000-088c793034c7d3c04cfb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0aor-4279500000-6c62b5fb61fc58056bf9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0cdm-4191000000-17150e51f1bb273c4ffa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-056r-0020900000-0d52fabc13f630dfec31 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052b-0291100000-aa18ba3164113c1e9fbe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05mk-5393000000-99af4d06909f5940a98d | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0034032 |
|---|
| FooDB ID | FDB012272 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 172668 |
|---|
| PubChem Compound ID | 85190792 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|