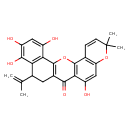| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:14:29 UTC |
|---|
| Update Date | 2016-11-09 01:18:37 UTC |
|---|
| Accession Number | CHEM028131 |
|---|
| Identification |
|---|
| Common Name | Artobiloxanthone |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 8,9-dihydro-6,10,11,13-Tetrahydroxy-3,3-dimethyl-9-(1-methylethenyl)-3H,7H-benzo[c]pyrano[3,2-H]xanthen-7-one, 9ci | HMDB | | KB 1 | HMDB | | Artobiloxanthone | MeSH |
|
|---|
| Chemical Formula | C25H22O7 |
|---|
| Average Molecular Mass | 434.438 g/mol |
|---|
| Monoisotopic Mass | 434.137 g/mol |
|---|
| CAS Registry Number | 121748-25-2 |
|---|
| IUPAC Name | 11,18,19,21-tetrahydroxy-7,7-dimethyl-16-(prop-1-en-2-yl)-2,8-dioxapentacyclo[12.8.0.0³,¹².0⁴,⁹.0¹⁷,²²]docosa-1(14),3(12),4(9),5,10,17(22),18,20-octaen-13-one |
|---|
| Traditional Name | 11,18,19,21-tetrahydroxy-7,7-dimethyl-16-(prop-1-en-2-yl)-2,8-dioxapentacyclo[12.8.0.0³,¹².0⁴,⁹.0¹⁷,²²]docosa-1(14),3(12),4(9),5,10,17(22),18,20-octaen-13-one |
|---|
| SMILES | CC(=C)C1CC2=C(OC3=C(C(O)=CC4=C3C=CC(C)(C)O4)C2=O)C2=C1C(O)=C(O)C=C2O |
|---|
| InChI Identifier | InChI=1S/C25H22O7/c1-10(2)12-7-13-21(29)20-15(27)9-17-11(5-6-25(3,4)32-17)23(20)31-24(13)19-14(26)8-16(28)22(30)18(12)19/h5-6,8-9,12,26-28,30H,1,7H2,2-4H3 |
|---|
| InChI Key | ZIYAGIMFLYOZDS-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyranoxanthones. These are organic aromatic compounds containing a pyran or a hydrogenated derivative fused to a xanthone ring system. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzopyrans |
|---|
| Sub Class | 1-benzopyrans |
|---|
| Direct Parent | Pyranoxanthones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyranoxanthone
- Naphthopyranone
- Naphthopyran
- Pyranochromene
- 2,2-dimethyl-1-benzopyran
- Chromone
- 1-naphthol
- Naphthalene
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Phenol
- Pyranone
- Benzenoid
- Pyran
- Vinylogous acid
- Heteroaromatic compound
- Oxacycle
- Ether
- Polyol
- Organic oxygen compound
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-066u-1232900000-e84a9cfc02616843c8d7 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-000i-1020139000-67d942d2ab4cee2e2bdd | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0003900000-668be1310d0d6fcb6285 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00p0-1109400000-39bbf898fd245c31f0b6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-02vi-4019000000-5c30c4c85f0d9c4feaf1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0000900000-c0372ac112ace47c9156 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0004900000-d71552c889e74c89b61b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-002b-0029100000-ac17fcf0d204d9708d44 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0000900000-8c5edb9d93203123adad | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0000900000-8c5edb9d93203123adad | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014r-0092500000-0f680dca4ab4f1c8f6b9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0000900000-81a03ca7359d4e6c5e6c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0000900000-81a03ca7359d4e6c5e6c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0api-0190200000-eca206f39d98b7f654e8 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00004104 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 24673270 |
|---|
| ChEBI ID | 172673 |
|---|
| PubChem Compound ID | 46887866 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|