| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:13:54 UTC |
|---|
| Update Date | 2016-11-09 01:18:36 UTC |
|---|
| Accession Number | CHEM028116 |
|---|
| Identification |
|---|
| Common Name | (E,E)-1-Chloro-3,11-tridecadiene-5,7,9-triyn-2-ol |
|---|
| Class | Small Molecule |
|---|
| Description | (E,E)-1-Chloro-3,11-tridecadiene-5,7,9-triyn-2-ol is found in fats and oils. (E,E)-1-Chloro-3,11-tridecadiene-5,7,9-triyn-2-ol is isolated from Carthamus tinctorius (safflower). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C13H11ClO |
|---|
| Average Molecular Mass | 218.679 g/mol |
|---|
| Monoisotopic Mass | 218.050 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (3E,11Z)-1-chlorotrideca-3,11-dien-5,7,9-triyn-2-ol |
|---|
| Traditional Name | (3E,11Z)-1-chlorotrideca-3,11-dien-5,7,9-triyn-2-ol |
|---|
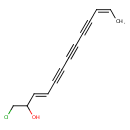
| SMILES | C\C=C/C#CC#CC#C\C=C\C(O)CCl |
|---|
| InChI Identifier | InChI=1S/C13H11ClO/c1-2-3-4-5-6-7-8-9-10-11-13(15)12-14/h2-3,10-11,13,15H,12H2,1H3/b3-2-,11-10+ |
|---|
| InChI Key | BWFFGWCCMCLGBQ-JSQGHLFOSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as chlorohydrins. These are alcohols substituted by a chlorine atom at a saturated carbon atom otherwise bearing only hydrogen or hydrocarbyl groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organohalogen compounds |
|---|
| Class | Halohydrins |
|---|
| Sub Class | Chlorohydrins |
|---|
| Direct Parent | Chlorohydrins |
|---|
| Alternative Parents | |
|---|
| Substituents | - Secondary alcohol
- Chlorohydrin
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Organochloride
- Alkyl halide
- Alkyl chloride
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000l-2900000000-5be7e508fbe7785c03fe | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-009l-9230000000-85e291ef9def47a8937d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uxr-0290000000-1aaa0889a1b9b9ab3c2c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-016r-4940000000-dd494a68c1dd92f9dae9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0v0c-9500000000-91db9066e9cc1d21ad74 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0290000000-ef255ea84e43ef481689 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-015a-0920000000-36b7557237f7184a07c6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-02d0-6900000000-dc71a4b4c282d0c88dc8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0190000000-3080194f1954cfd3eb4f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-9100000000-7f93922301798045b1f1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03ea-9600000000-4a1582eedb3674ce8f34 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-3190000000-50bae8a617816f40557a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01t9-9500000000-cfce7fda8f5635b57247 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-007a-9800000000-14d3fc459ad46e0b212f | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0034007 |
|---|
| FooDB ID | FDB012243 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35013685 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131751511 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|