| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:13:04 UTC |
|---|
| Update Date | 2016-11-09 01:18:36 UTC |
|---|
| Accession Number | CHEM028096 |
|---|
| Identification |
|---|
| Common Name | Demethylvestitol |
|---|
| Class | Small Molecule |
|---|
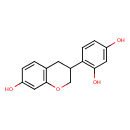
| Description | Demethylvestitol is found in common bean. Demethylvestitol is isolated from Lablab niger (hyacinth bean) and Phaseolus vulgaris (kidney bean). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Pipecuronium | MeSH | | Pipecuronium dibromide, (17 alpha)-isomer | MeSH | | Pipecuronium dibromide, (3 beta)-isomer | MeSH | | Pipecuronium dibromide, dihydrate | MeSH | | Pipecurium | MeSH | | Bromide, pipecurium | MeSH | | Bromide, pipecuronium | MeSH | | Pipecuronium bromide | MeSH | | Pipecuronium dibromide, (16 alpha)-isomer | MeSH | | Arduan | MeSH | | Dibromide pipecuronium | MeSH | | Dibromide, dihydrate pipecuronium | MeSH | | Dihydrate pipecuronium dibromide | MeSH | | Pipecurium bromide | MeSH | | Pipecuronium, dibromide | MeSH | | 3,4-dihydro-3-(2,4-Dihydroxyphenyl)-7-hydroxy-2H-1-benzopyran | HMDB | | 4-(3,4-dihydro-7-Hydroxy-2H-1-benzopyran-4-yl)-1,3-benzenediol, 9ci | HMDB | | 7,2',4'-Trihydroxyisoflavan | HMDB | | pipecuronio Bromuro | HMDB |
|
|---|
| Chemical Formula | C15H14O4 |
|---|
| Average Molecular Mass | 258.269 g/mol |
|---|
| Monoisotopic Mass | 258.089 g/mol |
|---|
| CAS Registry Number | 65332-45-8 |
|---|
| IUPAC Name | 4-(7-hydroxy-3,4-dihydro-2H-1-benzopyran-3-yl)benzene-1,3-diol |
|---|
| Traditional Name | demethylvestitol |
|---|
| SMILES | OC1=CC(O)=C(C=C1)C1COC2=C(C1)C=CC(O)=C2 |
|---|
| InChI Identifier | InChI=1S/C15H14O4/c16-11-3-4-13(14(18)6-11)10-5-9-1-2-12(17)7-15(9)19-8-10/h1-4,6-7,10,16-18H,5,8H2 |
|---|
| InChI Key | CJZBXHPHEBCWLV-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as isoflavanols. These are polycyclic compounds containing a hydroxylated isoflavan skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Isoflavonoids |
|---|
| Sub Class | Isoflavans |
|---|
| Direct Parent | Isoflavanols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Isoflavanol
- Hydroxyisoflavonoid
- Chromane
- Benzopyran
- 1-benzopyran
- Resorcinol
- Alkyl aryl ether
- Phenol
- 1-hydroxy-2-unsubstituted benzenoid
- 1-hydroxy-4-unsubstituted benzenoid
- Benzenoid
- Monocyclic benzene moiety
- Organoheterocyclic compound
- Ether
- Oxacycle
- Hydrocarbon derivative
- Organooxygen compound
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0553-0590000000-2c0bca31092dfea212e7 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-0zmi-3022900000-4539bb07bfffda37b174 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ab9-0890000000-feebc814b570d1822973 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dl-0950000000-8613c9fd6e87aaa46cfc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05fr-3920000000-28cde73d029166d04627 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0390000000-bc72e3f53044527a2755 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0980000000-5c07eb85c5df3850f182 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ab9-3910000000-cdd6f51494588e40ce3b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4j-0590000000-0fc888d544d19b178a04 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4j-0980000000-ff6cd3ec3ced984c021f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00ri-4920000000-88560d7baa6e7c78b330 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0290000000-31d1297674ece6f611b5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0690000000-88623afb300c6aaee822 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01wr-0910000000-e1ac3be06a5cc42b713f | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0033986 |
|---|
| FooDB ID | FDB012218 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00009708 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 509323 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 585939 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|