| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:12:34 UTC |
|---|
| Update Date | 2016-11-09 01:18:36 UTC |
|---|
| Accession Number | CHEM028083 |
|---|
| Identification |
|---|
| Common Name | (R)-Pantothenic acid 4'-O-b-D-glucoside |
|---|
| Class | Small Molecule |
|---|
| Description | (R)-Pantothenic acid 4'-O-b-D-glucoside is found in garden tomato. (R)-Pantothenic acid 4'-O-b-D-glucoside is isolated from tomato juice. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
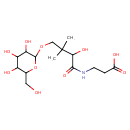
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (R)-Pantothenate 4'-O-b-D-glucoside | Generator | | 3-[(1,2-Dihydroxy-3,3-dimethyl-4-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}butylidene)amino]propanoate | Generator |
|
|---|
| Chemical Formula | C15H27NO10 |
|---|
| Average Molecular Mass | 381.376 g/mol |
|---|
| Monoisotopic Mass | 381.163 g/mol |
|---|
| CAS Registry Number | 29493-59-2 |
|---|
| IUPAC Name | 3-(2-hydroxy-3,3-dimethyl-4-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}butanamido)propanoic acid |
|---|
| Traditional Name | 3-(2-hydroxy-3,3-dimethyl-4-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}butanamido)propanoic acid |
|---|
| SMILES | CC(C)(COC1OC(CO)C(O)C(O)C1O)C(O)C(=O)NCCC(O)=O |
|---|
| InChI Identifier | InChI=1S/C15H27NO10/c1-15(2,12(23)13(24)16-4-3-8(18)19)6-25-14-11(22)10(21)9(20)7(5-17)26-14/h7,9-12,14,17,20-23H,3-6H2,1-2H3,(H,16,24)(H,18,19) |
|---|
| InChI Key | GMURXYJSTRMISD-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as fatty acyl glycosides of mono- and disaccharides. Fatty acyl glycosides of mono- and disaccharides are compounds composed of a mono- or disaccharide moiety linked to one hydroxyl group of a fatty alcohol or of a phosphorylated alcohol (phosphoprenols), a hydroxy fatty acid or to one carboxyl group of a fatty acid (ester linkage) or to an amino alcohol. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acyl glycosides |
|---|
| Direct Parent | Fatty acyl glycosides of mono- and disaccharides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Fatty acyl glycoside of mono- or disaccharide
- Alkyl glycoside
- Hexose monosaccharide
- Glycosyl compound
- O-glycosyl compound
- Monosaccharide
- Oxane
- Secondary alcohol
- Acetal
- Carboximidic acid
- Carboximidic acid derivative
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Organic oxide
- Alcohol
- Hydrocarbon derivative
- Organonitrogen compound
- Organooxygen compound
- Organic nitrogen compound
- Carbonyl group
- Primary alcohol
- Organic oxygen compound
- Organopnictogen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0ir0-7849000000-d80cb4a86b7d5cabd17d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-0udi-4200259000-11ea728fc8f9b60ef89b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-9124000000-ebee20832bc1d1fe527d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0079-9120000000-b1196228766ac233fae3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0076-9000000000-ec678741aaff18af7203 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01q9-3559000000-2bed5ac605385982142c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-7932000000-538ef138b162f33efc6e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000f-9410000000-581eeed96122b3ee8c4e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0fef-7297000000-729e14f767d20ce6c670 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uk9-9381000000-c6bf519fcf229e0c4323 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-9240000000-587e98630e495d7fd01d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0019000000-0bbe5d9c6bda5de9af7a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-029x-7196000000-9c92a9237b82a39c4d45 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fki-9410000000-8419f3adf4e8655e958d | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0033965 |
|---|
| FooDB ID | FDB012194 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 168031 |
|---|
| PubChem Compound ID | 14132229 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|