| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:12:09 UTC |
|---|
| Update Date | 2016-11-09 01:18:36 UTC |
|---|
| Accession Number | CHEM028075 |
|---|
| Identification |
|---|
| Common Name | Cyclosquamosin A |
|---|
| Class | Small Molecule |
|---|
| Description | Constituent of the seeds of Annona squamosa (sugar apple). Cyclosquamosin A is found in fruits. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
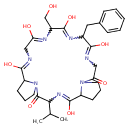
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C31H43N7O8 |
|---|
| Average Molecular Mass | 641.726 g/mol |
|---|
| Monoisotopic Mass | 641.317 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 15-benzyl-18-(hydroxymethyl)-3-(propan-2-yl)-1,4,10,13,16,19,22-heptaazatricyclo[22.3.0.0⁶,¹⁰]heptacosane-2,5,11,14,17,20,23-heptone |
|---|
| Traditional Name | 15-benzyl-18-(hydroxymethyl)-3-isopropyl-1,4,10,13,16,19,22-heptaazatricyclo[22.3.0.0⁶,¹⁰]heptacosane-2,5,11,14,17,20,23-heptone |
|---|
| SMILES | CC(C)C1NC(=O)C2CCCN2C(=O)CNC(=O)C(CC2=CC=CC=C2)NC(=O)C(CO)NC(=O)CNC(=O)C2CCCN2C1=O |
|---|
| InChI Identifier | InChI=1S/C31H43N7O8/c1-18(2)26-31(46)38-13-7-10-22(38)29(44)32-15-24(40)34-21(17-39)28(43)35-20(14-19-8-4-3-5-9-19)27(42)33-16-25(41)37-12-6-11-23(37)30(45)36-26/h3-5,8-9,18,20-23,26,39H,6-7,10-17H2,1-2H3,(H,32,44)(H,33,42)(H,34,40)(H,35,43)(H,36,45) |
|---|
| InChI Key | MRKIEHJOWYDIFL-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as oligopeptides. These are organic compounds containing a sequence of between three and ten alpha-amino acids joined by peptide bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Oligopeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-oligopeptide
- Cyclic alpha peptide
- Macrolactam
- Alpha-amino acid or derivatives
- Monocyclic benzene moiety
- Benzenoid
- Pyrrolidine
- Tertiary carboxylic acid amide
- Secondary carboxylic acid amide
- Lactam
- Carboxamide group
- Organoheterocyclic compound
- Azacycle
- Hydrocarbon derivative
- Organic nitrogen compound
- Alcohol
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Organic oxide
- Organopnictogen compound
- Carbonyl group
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00dl-1111019000-eda5faf0b488acfa89ab | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00xr-7826819000-689dbc1e1d73f1884871 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-066r-9711000000-98faafd4022f0ba88dc9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0005-7001189000-5cc384e6613d79281343 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9314285000-9bfbd2f1c458ef648227 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0pi4-9541110000-8e75774ff42c7fdd3e1d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0000009000-7f75d870f6acd68385af | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0000009000-cdd73d32bc67e66c0156 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-e3902423d50f2f27b183 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0000009000-a1f6a64b2f4f53d0d3f8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0000009000-731a122f52f15610957b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-8000092000-d6e654790b1f693b7297 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | FDB012179 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 73194485 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|