| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:10:24 UTC |
|---|
| Update Date | 2016-11-09 01:18:35 UTC |
|---|
| Accession Number | CHEM028032 |
|---|
| Identification |
|---|
| Common Name | Solanidine |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
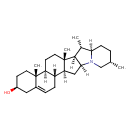
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (-)-Solanidine | ChEBI | | (22R,25S)-Solanidanine | ChEBI | | (22R,25S)-Solanidine | ChEBI | | (2S,4AR,4BS,6as,6BR,7S,7ar,10S,12as,13as,13BS)-4a,6a,7,10-tetramethyl-2,3,4,4a,4b,5,6,6a,6b,7,7a,8,9,10,11,12a,13,13a,13b,14-icosahydro-1H-naphtho[2',1':4,5]indeno[1,2-b]indolizin-2-ol | ChEBI | | (3beta)-Solanid-5-en-3-ol | ChEBI | | 3-beta-Solanid-5-en-3-ol | ChEBI | | Solanid-5-en-3-beta-ol | ChEBI | | Solanidin | ChEBI | | Solatubin | ChEBI | | Solatubine | ChEBI | | (3b)-Solanid-5-en-3-ol | Generator | | (3Β)-solanid-5-en-3-ol | Generator | | 3-b-Solanid-5-en-3-ol | Generator | | 3-Β-solanid-5-en-3-ol | Generator | | Solanid-5-en-3-b-ol | Generator | | Solanid-5-en-3-β-ol | Generator | | Solanid-5-en-3beta-ol | HMDB | | Solanid-5-en-3b-ol | HMDB | | Solanid-5-en-3β-ol | HMDB | | 22R,25S-Solanidanine | HMDB | | 22R,25S-Solanidine | HMDB | | 3-b-Solanid-5-en-3-ol(9CL) | HMDB | | 3-beta-Solanid-5-en-3-ol(9CL) | HMDB | | Solanid-5-en-3-ol | HMDB | | Solanid-5-en-3-ol (acd/name 4.0) | HMDB | | Solanid-5-en-3.beta.-ol | HMDB | | Solanid-5-en-3beta-ol(8ci) | HMDB |
|
|---|
| Chemical Formula | C27H43NO |
|---|
| Average Molecular Mass | 397.636 g/mol |
|---|
| Monoisotopic Mass | 397.334 g/mol |
|---|
| CAS Registry Number | 80-78-4 |
|---|
| IUPAC Name | (1S,2S,7S,10R,11S,14S,15R,16S,17R,20S,23S)-10,14,16,20-tetramethyl-22-azahexacyclo[12.10.0.0²,¹¹.0⁵,¹⁰.0¹⁵,²³.0¹⁷,²²]tetracos-4-en-7-ol |
|---|
| Traditional Name | solanidine |
|---|
| SMILES | CC1C2CCC(C)CN2C2CC3C4CC=C5CC(O)CCC5(C)C4CCC3(C)C12 |
|---|
| InChI Identifier | InChI=1S/C27H43NO/c1-16-5-8-23-17(2)25-24(28(23)15-16)14-22-20-7-6-18-13-19(29)9-11-26(18,3)21(20)10-12-27(22,25)4/h6,16-17,19-25,29H,5,7-15H2,1-4H3 |
|---|
| InChI Key | JVKYZPBMZPJNAJ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as solanidines and derivatives. These are steroids with a structure based on the solanidane skeleton. Solanidane arises from the conversion of a cholestane side-chain into a bicyclic system. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Steroidal alkaloids |
|---|
| Direct Parent | Solanidines and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Solanidane skeleton
- 3-hydroxy-delta-5-steroid
- 3-hydroxysteroid
- Hydroxysteroid
- 3-beta-hydroxysteroid
- 3-beta-hydroxy-delta-5-steroid
- Azasteroid
- Delta-5-steroid
- Alkaloid or derivatives
- Indolizidine
- N-alkylpyrrolidine
- Piperidine
- Cyclic alcohol
- Pyrrolidine
- Tertiary aliphatic amine
- Tertiary amine
- Secondary alcohol
- Organoheterocyclic compound
- Azacycle
- Organonitrogen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Organopnictogen compound
- Amine
- Organooxygen compound
- Organic oxygen compound
- Alcohol
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00lr-0119000000-09758005bcbc04782ff4 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0udl-1213900000-9122bd157f18325b320b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001j-0009000000-5b0fb24be88683d068b5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001j-0139000000-911def26f967b66af0fa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0wti-1159000000-e91adb85d8cc7c4803c1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0009000000-b7424d5bd5a12fdf98d9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0009000000-68f4c5d2355e9a863c72 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-003u-5009000000-1267a2feb79a6babccfd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0009000000-9adef56b5a2ae287a34c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0009000000-9adef56b5a2ae287a34c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-0009000000-7801b00b472dd0fa1eca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0009000000-99dc14115be2ec86a844 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0139000000-2f8e89fdf0e2e16b8222 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-002b-5921000000-d4f1d4372fb415196928 | Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0udi-5910000000-da12df0b66b01e498980 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0003236 |
|---|
| FooDB ID | FDB012098 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00002261 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | CPD-9217 |
|---|
| METLIN ID | 3517 |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Solanidine |
|---|
| Chemspider ID | 59150 |
|---|
| ChEBI ID | 28374 |
|---|
| PubChem Compound ID | 65727 |
|---|
| Kegg Compound ID | C06543 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|