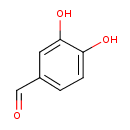
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-052r-2900000000-fd0d28650a72226166e6 | Spectrum |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00yi-8690000000-16ccc54dcf22361cffb1 | Spectrum |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - ESI-TOF 10V, Negative | splash10-0002-0040900010-522c055741661bbc5ba8 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - ESI-TOF 20V, Negative | splash10-0002-0040900010-522c055741661bbc5ba8 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - ESI-TOF , Negative | splash10-0002-0040900010-522c055741661bbc5ba8 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - ESI-TOF 10V, Negative | splash10-002r-0905000000-7987816fb8648b0eea18 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - ESI-TOF 10V, Negative | splash10-000i-0900000000-aa91c9c469ec39450435 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - ESI-TOF 20V, Negative | splash10-000i-0900000000-25b5b2bc87af9df04e91 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - ESI-TOF , Negative | splash10-000i-0900000000-03d9e2dbe57c9bcfb9ab | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - ESI-TOF 10V, Negative | splash10-002r-0905000000-7987816fb8648b0eea18 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-TOF , negative | splash10-000i-0900000000-aa91c9c469ec39450435 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-TOF , negative | splash10-000i-0900000000-25b5b2bc87af9df04e91 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0900000000-aa91c9c469ec39450435 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0900000000-25b5b2bc87af9df04e91 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0900000000-db13978cfcbc33c6da32 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-1900000000-ed3a0258f14e4361b25c | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pb9-9200000000-cdb54c91531419396213 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0900000000-db13978cfcbc33c6da32 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-1900000000-ed3a0258f14e4361b25c | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pb9-9200000000-cdb54c91531419396213 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0900000000-882b8375384be94a3491 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-1900000000-25b2a170eaf9dd74c4a0 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0kw4-9300000000-e349b638bc98dcdf0ade | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0900000000-882b8375384be94a3491 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-1900000000-25b2a170eaf9dd74c4a0 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0kw4-9300000000-e349b638bc98dcdf0ade | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |