| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:05:43 UTC |
|---|
| Update Date | 2016-11-09 01:18:34 UTC |
|---|
| Accession Number | CHEM027930 |
|---|
| Identification |
|---|
| Common Name | Elemicin |
|---|
| Class | Small Molecule |
|---|
| Description | Elemicin is found in carrot. Elemicin is a constituent of Elemi oil and Myristica fragrans (nutmeg). |
|---|
| Contaminant Sources | - FooDB Chemicals
- Tobacco Smoke Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
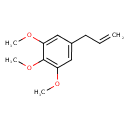
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 5'-Metoxy eugenol | ChEMBL, HMDB | | 1,2,3-Trimethoxy-5-(2-propenyl)-benzene | HMDB | | 1,2,3-Trimethoxy-5-(2-propenyl)benzene, 9ci | HMDB | | 1,2,3-Trimethoxy-5-allylbenzene (elemicin) | HMDB | | 1,2,3-Trimethoxy-5-[2-propenyl]-benzene | HMDB | | 3,4, 5-Trimethoxyallylbenzene | HMDB | | 3,4,5-Trimethoxyallylbenzene | HMDB | | 4-Allyl-1,2,6-trimethoxybenzene | HMDB | | 5-Allyl-1,2,3-trimethoxy-benzene | HMDB | | 5-Allyl-1,2,3-trimethoxybenzene | HMDB | | Benzene, 1,2,3-trimethoxy-5-(2-propenyl)- (9ci) | HMDB | | Benzene, 5-(2-propenyl)-1,2,3-trimethoxy | HMDB | | Elemicine | HMDB |
|
|---|
| Chemical Formula | C12H16O3 |
|---|
| Average Molecular Mass | 208.254 g/mol |
|---|
| Monoisotopic Mass | 208.110 g/mol |
|---|
| CAS Registry Number | 487-11-6 |
|---|
| IUPAC Name | 1,2,3-trimethoxy-5-(prop-2-en-1-yl)benzene |
|---|
| Traditional Name | elemicin |
|---|
| SMILES | COC1=CC(CC=C)=CC(OC)=C1OC |
|---|
| InChI Identifier | InChI=1S/C12H16O3/c1-5-6-9-7-10(13-2)12(15-4)11(8-9)14-3/h5,7-8H,1,6H2,2-4H3 |
|---|
| InChI Key | BPLQKQKXWHCZSS-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as anisoles. These are organic compounds containing a methoxybenzene or a derivative thereof. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Phenol ethers |
|---|
| Sub Class | Anisoles |
|---|
| Direct Parent | Anisoles |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenoxy compound
- Methoxybenzene
- Anisole
- Alkyl aryl ether
- Monocyclic benzene moiety
- Ether
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-054o-1910000000-90f58b5ac3c420a0bead | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0190000000-0d12e8d4c75330b0079d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-3790000000-c789d6a44beb86af1b56 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-5900000000-f5bd2ff33bc271ebe936 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0090000000-445d52139886db9fa3a1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0890000000-b4607d26dffb40f5f337 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0cdr-3900000000-9daed5f20cf190665c47 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0090000000-3ac4b42a0e1a385b89e7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0890000000-a89a807497bbc1eb67ea | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014l-9300000000-667bbc7580de5f67c153 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0090000000-65fdf834be31e8e78310 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a6r-0950000000-27c93822d629f9183bb2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0cdi-9520000000-359f70ef5bc549d05903 | Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0a4l-4950000000-139934290bd7ee24b6ba | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0033778 |
|---|
| FooDB ID | FDB011932 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00002739 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Elemicin |
|---|
| Chemspider ID | 9830 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 10248 |
|---|
| Kegg Compound ID | C10451 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|