| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:04:53 UTC |
|---|
| Update Date | 2016-11-09 01:18:34 UTC |
|---|
| Accession Number | CHEM027914 |
|---|
| Identification |
|---|
| Common Name | Marcanine A |
|---|
| Class | Small Molecule |
|---|
| Description | Marcanine A is found in alcoholic beverages. Marcanine A is an alkaloid from Annona glabra (pond apple). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
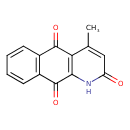
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Griffiazanone b | MeSH | | 4-Methyl-1H-1-aza-2,9,10-anthracenetrione | HMDB | | 4-methylbenzo[g]Quinoline-2,5,10(1H)-trione, 9ci | HMDB | | Marcanine a | MeSH |
|
|---|
| Chemical Formula | C14H9NO3 |
|---|
| Average Molecular Mass | 239.226 g/mol |
|---|
| Monoisotopic Mass | 239.058 g/mol |
|---|
| CAS Registry Number | 157463-84-8 |
|---|
| IUPAC Name | 4-methyl-1H,2H,5H,10H-benzo[g]quinoline-2,5,10-trione |
|---|
| Traditional Name | marcanin A |
|---|
| SMILES | CC1=CC(=O)NC2=C1C(=O)C1=C(C=CC=C1)C2=O |
|---|
| InChI Identifier | InChI=1S/C14H9NO3/c1-7-6-10(16)15-12-11(7)13(17)8-4-2-3-5-9(8)14(12)18/h2-6H,1H3,(H,15,16) |
|---|
| InChI Key | GYAHTYNHCVTZOK-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzoquinolines. These are organic compounds containing a benzene fused to a quinoline ring system. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Quinolines and derivatives |
|---|
| Sub Class | Benzoquinolines |
|---|
| Direct Parent | Benzoquinolines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Benzoquinoline
- Quinoline quinone
- Naphthalene
- Aryl ketone
- Methylpyridine
- Pyridinone
- Pyridine
- Benzenoid
- Heteroaromatic compound
- Vinylogous amide
- Ketone
- Lactam
- Azacycle
- Organic nitrogen compound
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01p9-1980000000-9ac6b281dab43cf05a7d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0090000000-a972810102c1214918a3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0390000000-2b51265f3ec8bcc1ebeb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pi9-5930000000-45c4899a3c4498110060 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-e17ad508c39e20b27a2c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0090000000-f7f6843d6a883f0626a6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00ej-5980000000-80cd6c86544d798fbb2e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0090000000-9fa7debb10e1f3436126 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0090000000-f5e1477749b003df9c53 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0r0s-3930000000-3c35b44aeb6addc3a864 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-f79f832e3721a47c5ba2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0090000000-f79f832e3721a47c5ba2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-007a-0590000000-d153029c5429f8e0191f | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0033757 |
|---|
| FooDB ID | FDB011892 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00028313 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 8281180 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 10105653 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|