| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:01:57 UTC |
|---|
| Update Date | 2016-11-09 01:18:32 UTC |
|---|
| Accession Number | CHEM027864 |
|---|
| Identification |
|---|
| Common Name | Poppy acid |
|---|
| Class | Small Molecule |
|---|
| Description | Poppy acid occurs in opium (Papaver somniferum) and other Papaver species. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
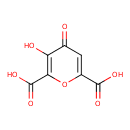
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Poppy acid | KEGG | | 3-Hydroxy-4-oxopyran-2,6-dicarboxylate | Generator, HMDB | | 3-Hydroxy-4-oxo-4H-pyran-2,6-dicarboxylic acid | HMDB | | 3-Hydroxy-4-pyrone-2,6-dicarboxylic acid | HMDB | | Meconic acid | HMDB | | Opium acid | HMDB | | Meconate | Generator |
|
|---|
| Chemical Formula | C7H4O7 |
|---|
| Average Molecular Mass | 200.103 g/mol |
|---|
| Monoisotopic Mass | 199.996 g/mol |
|---|
| CAS Registry Number | 497-59-6 |
|---|
| IUPAC Name | 3-hydroxy-4-oxo-4H-pyran-2,6-dicarboxylic acid |
|---|
| Traditional Name | 3-hydroxy-4-oxopyran-2,6-dicarboxylic acid |
|---|
| SMILES | OC(=O)C1=CC(=O)C(O)=C(O1)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C7H4O7/c8-2-1-3(6(10)11)14-5(4(2)9)7(12)13/h1,9H,(H,10,11)(H,12,13) |
|---|
| InChI Key | ZEGRKMXCOCRTCS-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyranones and derivatives. Pyranones and derivatives are compounds containing a pyran ring which bears a ketone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyrans |
|---|
| Sub Class | Pyranones and derivatives |
|---|
| Direct Parent | Pyranones and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyranone
- Dicarboxylic acid or derivatives
- Heteroaromatic compound
- Vinylogous acid
- Cyclic ketone
- Oxacycle
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0561-4900000000-f5e8bbaabc7cec73820a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-00dm-9347200000-fe35c5a7d7c550a845a6 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ue9-0890000000-725ed2fb6cffdc5b8c9a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f89-0940000000-30e0d5ee24bef5e44a6c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-066r-9500000000-8ff6afe220048a689475 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-602800576ee9d4604928 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-1900000000-d07716f0fb1c802add0f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03yi-9600000000-63616e78fb350b3583de | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0ik9-0900000000-85a39ff4758407ec8832 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0900000000-668a8ae5670aa7a819ad | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0w29-9500000000-08d5dbf8c2b0ca7ba0af | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001r-0900000000-cf81589bfc69f01262cd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0019-0910000000-43c11e3a5468446b10f8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0540-9000000000-747a064f4e27732823d8 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0033695 |
|---|
| FooDB ID | FDB011804 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00051519 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Meconic acid |
|---|
| Chemspider ID | 10465112 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 10347 |
|---|
| Kegg Compound ID | C20209 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|