| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:00:44 UTC |
|---|
| Update Date | 2016-11-09 01:18:32 UTC |
|---|
| Accession Number | CHEM027834 |
|---|
| Identification |
|---|
| Common Name | De-O-methyldihydrosterigmatocystin |
|---|
| Class | Small Molecule |
|---|
| Description | Mycotoxin production by Aspergillus versicolor. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
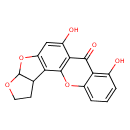
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Demethyldihydrosterigmatocystin | HMDB | | Dihydrodemethylsterigmatocystin | HMDB |
|
|---|
| Chemical Formula | C17H12O6 |
|---|
| Average Molecular Mass | 312.274 g/mol |
|---|
| Monoisotopic Mass | 312.063 g/mol |
|---|
| CAS Registry Number | 30517-66-9 |
|---|
| IUPAC Name | 11,15-dihydroxy-6,8,20-trioxapentacyclo[10.8.0.0²,⁹.0³,⁷.0¹⁴,¹⁹]icosa-1(12),2(9),10,14,16,18-hexaen-13-one |
|---|
| Traditional Name | 11,15-dihydroxy-6,8,20-trioxapentacyclo[10.8.0.0²,⁹.0³,⁷.0¹⁴,¹⁹]icosa-1(12),2(9),10,14,16,18-hexaen-13-one |
|---|
| SMILES | OC1=CC2=C(C3CCOC3O2)C2=C1C(=O)C1=C(O)C=CC=C1O2 |
|---|
| InChI Identifier | InChI=1S/C17H12O6/c18-8-2-1-3-10-13(8)15(20)14-9(19)6-11-12(16(14)22-10)7-4-5-21-17(7)23-11/h1-3,6-7,17-19H,4-5H2 |
|---|
| InChI Key | WUSMTEDKVPWFDN-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as sterigmatocystins. These are a group of closely related fungal metabolites chemically characterized by a xanthone moiety fused to a dihydrodifurano or a tetrahydrodifurano moiety. The chemical difference among the various sterigmagocystins are the presence or absence of unsaturation at positions 2 and 3 of the difurano ring system, the substitution pattern on positions 6, 7, and 10 of the xanthone system and/or the substituent on position 3 of the difurano system. They are produced by Aspergilus spp. and Bipolaris spp. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Sterigmatocystins |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Sterigmatocystins |
|---|
| Alternative Parents | |
|---|
| Substituents | - Sterigmatocystin backbone
- Xanthone
- Dibenzopyran
- Xanthene
- Chromone
- Benzopyran
- 1-benzopyran
- Coumaran
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Pyranone
- Pyran
- Benzenoid
- Heteroaromatic compound
- Tetrahydrofuran
- Vinylogous acid
- Oxacycle
- Acetal
- Organoheterocyclic compound
- Hydrocarbon derivative
- Organic oxide
- Organooxygen compound
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-001i-1190000000-333fc0c025f1a0991340 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00e9-4504900000-8ec5367a9cfefcd1ddae | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0029000000-68b36ea43dfc30c09a62 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0089000000-cf4f2b4c6375ec0ef1c1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01ta-1490000000-30ce98d0cdc6ae50dda8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0009000000-fdfe47d21a035416c9dc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0139000000-f9058f433c1086d6f0ca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00ou-1390000000-6148302a8c000974ec2e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0009000000-fbd933023572de11abf0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0009000000-fbd933023572de11abf0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01ow-0591000000-4fa22d9ff53e113d690c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0009000000-b504eaff11e6208b17b1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0049000000-3b842f4e2555c65afd0f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-029x-1390000000-59c8649686b037aa2c65 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0033658 |
|---|
| FooDB ID | FDB011760 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4588953 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 5486528 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|