| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:00:28 UTC |
|---|
| Update Date | 2016-11-09 01:18:32 UTC |
|---|
| Accession Number | CHEM027825 |
|---|
| Identification |
|---|
| Common Name | O-Demethylfonsecin |
|---|
| Class | Small Molecule |
|---|
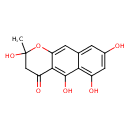
| Description | A heptaketide that is 2,3-dihydro-4H-benzo[g]chromen-4-one bearing a methyl substituent at position 2 and four hydroxy substituents at positions 2, 5, 6 and 8. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Demethylfonsecin | ChEBI | | Desmethylfonsecin | ChEBI | | De-O-methylfonsecin | HMDB | | YWA 1 | HMDB | | O-Demethylfonsecin | ChEBI |
|
|---|
| Chemical Formula | C14H12O6 |
|---|
| Average Molecular Mass | 276.242 g/mol |
|---|
| Monoisotopic Mass | 276.063 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 2,5,6,8-tetrahydroxy-2-methyl-2H,3H,4H-naphtho[2,3-b]pyran-4-one |
|---|
| Traditional Name | 2,5,6,8-tetrahydroxy-2-methyl-3H-naphtho[2,3-b]pyran-4-one |
|---|
| SMILES | CC1(O)CC(=O)C2=C(O)C3=C(O)C=C(O)C=C3C=C2O1 |
|---|
| InChI Identifier | InChI=1S/C14H12O6/c1-14(19)5-9(17)12-10(20-14)3-6-2-7(15)4-8(16)11(6)13(12)18/h2-4,15-16,18-19H,5H2,1H3 |
|---|
| InChI Key | RTYDQIKVNMHQMZ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as naphthopyranones. Naphthopyranones are compounds containing a naphthopyran skeleton where a ring carbon bears a carboxylic acid group. Naphthtopyran is made up of the pyran ring fused to a naphthalene ring system. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Naphthopyrans |
|---|
| Sub Class | Naphthopyranones |
|---|
| Direct Parent | Naphthopyranones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Benzochromone
- Naphthopyranone
- Chromone
- 2-naphthol
- 1-naphthol
- Chromane
- Benzopyran
- Naphthalene
- 1-benzopyran
- Aryl alkyl ketone
- Aryl ketone
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Pyranone
- Benzenoid
- Pyran
- Vinylogous acid
- Ketone
- Hemiacetal
- Polyol
- Oxacycle
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-053u-2190000000-92067630613a090a6268 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-00rg-9100740000-883c543f5e40f4da387c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-056r-0090000000-d4ed37b50af2d0f4561c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a6r-1090000000-57b3333d9a3ed9489239 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0gdi-1890000000-95593eff745cf0e03969 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0390000000-e0861a6bb40d34035f29 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-056r-0490000000-745be75b0f78defaf226 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00xr-1930000000-85766bd5d383df5fa4af | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0090000000-fdefbfcb4d27fe320d4b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0090000000-34fc5131036b1ec5e26f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0aou-3290000000-3468e25876a93af9e730 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0090000000-9f46e36098b5d5b4738d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-056r-0090000000-f42f9726e3904deb0d4b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05mo-5290000000-38b1ad796f7ab2088653 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0033649 |
|---|
| FooDB ID | FDB011751 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | CPD-18255 |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35013649 |
|---|
| ChEBI ID | 133763 |
|---|
| PubChem Compound ID | 100923872 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|