| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 23:59:20 UTC |
|---|
| Update Date | 2016-11-09 01:18:32 UTC |
|---|
| Accession Number | CHEM027798 |
|---|
| Identification |
|---|
| Common Name | Zearalenone 4-sulfate |
|---|
| Class | Small Molecule |
|---|
| Description | Zearalenone 4-sulfate is produced by Fusarium graminearum grown on rice. Estrogenic mycotoxin. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
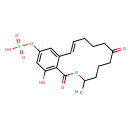
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Zearalenone 4-sulfuric acid | Generator | | Zearalenone 4-sulphate | Generator | | Zearalenone 4-sulphuric acid | Generator | | Zearalenone-4-sulfate | HMDB | | Zearalenone-4-sulphate | HMDB | | (16-Hydroxy-3-methyl-1,7-dioxo-3,4,5,6,7,8,9,10-octahydro-1H-2-benzoxacyclotetradecin-14-yl)oxidanesulfonate | HMDB | | (16-Hydroxy-3-methyl-1,7-dioxo-3,4,5,6,7,8,9,10-octahydro-1H-2-benzoxacyclotetradecin-14-yl)oxidanesulphonate | HMDB | | (16-Hydroxy-3-methyl-1,7-dioxo-3,4,5,6,7,8,9,10-octahydro-1H-2-benzoxacyclotetradecin-14-yl)oxidanesulphonic acid | HMDB |
|
|---|
| Chemical Formula | C18H22O8S |
|---|
| Average Molecular Mass | 398.427 g/mol |
|---|
| Monoisotopic Mass | 398.104 g/mol |
|---|
| CAS Registry Number | 132505-04-5 |
|---|
| IUPAC Name | (16-hydroxy-3-methyl-1,7-dioxo-3,4,5,6,7,8,9,10-octahydro-1H-2-benzoxacyclotetradecin-14-yl)oxidanesulfonic acid |
|---|
| Traditional Name | (16-hydroxy-3-methyl-1,7-dioxo-4,5,6,8,9,10-hexahydro-3H-2-benzoxacyclotetradecin-14-yl)oxidanesulfonic acid |
|---|
| SMILES | CC1CCCC(=O)CCC\C=C\C2=CC(OS(O)(=O)=O)=CC(O)=C2C(=O)O1 |
|---|
| InChI Identifier | InChI=1S/C18H22O8S/c1-12-6-5-9-14(19)8-4-2-3-7-13-10-15(26-27(22,23)24)11-16(20)17(13)18(21)25-12/h3,7,10-12,20H,2,4-6,8-9H2,1H3,(H,22,23,24)/b7-3+ |
|---|
| InChI Key | GQAJNGUAQKYPCH-XVNBXDOJSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as zearalenones. These are macrolides which contains a fourteen-member lactone fused to 1,3-dihydroxybenzene. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Macrolides and analogues |
|---|
| Sub Class | Zearalenones |
|---|
| Direct Parent | Zearalenones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Zearalenone-skeleton
- Arylsulfate
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Sulfuric acid monoester
- Benzenoid
- Sulfuric acid ester
- Sulfate-ester
- Organic sulfuric acid or derivatives
- Vinylogous acid
- Carboxylic acid ester
- Ketone
- Lactone
- Cyclic ketone
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Oxacycle
- Organoheterocyclic compound
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Organooxygen compound
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0159-0029000000-bd2c9dd9781d93aeecdb | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-074j-4209400000-6e450ed33e7fae36f95e | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0009000000-bf57d3f04996dc81334d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0gc0-3109000000-6136771fa1fffe452f8c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0bu1-9241000000-dbfb030038afcc3931ad | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0009000000-5431eb99b5105f704795 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014j-0019000000-6b5f29a985b4a844e9d8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01pt-3091000000-eae025faf1a2e6aec022 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0009000000-767631fa716e2ed066ee | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-6009000000-6e7527a2143a4c5af67f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-9010000000-ce948dc2dd97fdc309e8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001j-0009000000-3ab8824ed2a266772ebc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0009000000-01da166699e351791338 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-2091000000-be480fe198d4b0a3efa0 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0033623 |
|---|
| FooDB ID | FDB011711 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00057851 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 22369969 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 45359500 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|