| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 23:59:17 UTC |
|---|
| Update Date | 2016-11-09 01:18:32 UTC |
|---|
| Accession Number | CHEM027797 |
|---|
| Identification |
|---|
| Common Name | Cabbage identification factor 2 |
|---|
| Class | Small Molecule |
|---|
| Description | Constituent of Balsamita major (costmary). Alkhanol is found in tea and herbs and spices. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
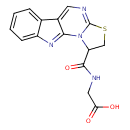
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Alhanol | HMDB | | Alkanol | HMDB | | Isoerivanin | HMDB | | [3S-(3alpha,3Aalpha,5abeta,6alpha,8alpha,9bbeta)]-3a,4,5,5a,6,7,8,9b-octahydro-6,8-dihydroxy-3,5a,9-trimethylnaphtho[1,2-b]furan-2(3H)-one | HMDB | | 2-{[hydroxy({5-thia-2,7,16-triazatetracyclo[7.7.0.0²,⁶.0¹⁰,¹⁵]hexadeca-1(16),6,8,10,12,14-hexaen-3-yl})methylidene]amino}acetate | Generator |
|
|---|
| Chemical Formula | C15H12N4O3S |
|---|
| Average Molecular Mass | 328.346 g/mol |
|---|
| Monoisotopic Mass | 328.063 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 2-{5-thia-2,7,16-triazatetracyclo[7.7.0.0²,⁶.0¹⁰,¹⁵]hexadeca-1(16),6,8,10,12,14-hexaen-3-ylformamido}acetic acid |
|---|
| Traditional Name | {5-thia-2,7,16-triazatetracyclo[7.7.0.0²,⁶.0¹⁰,¹⁵]hexadeca-1(16),6,8,10,12,14-hexaen-3-ylformamido}acetic acid |
|---|
| SMILES | OC(=O)CNC(=O)C1CSC2=NC=C3C(=NC4=CC=CC=C34)N12 |
|---|
| InChI Identifier | InChI=1S/C15H12N4O3S/c20-12(21)6-16-14(22)11-7-23-15-17-5-9-8-3-1-2-4-10(8)18-13(9)19(11)15/h1-5,11H,6-7H2,(H,16,22)(H,20,21) |
|---|
| InChI Key | ZPXJZOIRMZYIAP-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as eudesmanolides, secoeudesmanolides, and derivatives. These are terpenoids with a structure based on the eudesmanolide (a 3,5a,9-trimethyl-naphtho[1,2-b]furan-2-one derivative) or secoeudesmanolide (a 3,6-dimethyl-5-(pentan-2-yl)-1-benzofuran-2-one derivative) skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Terpene lactones |
|---|
| Direct Parent | Eudesmanolides, secoeudesmanolides, and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Eudesmanolide
- Sesquiterpenoid
- Naphthofuran
- Gamma butyrolactone
- Tetrahydrofuran
- Secondary alcohol
- Lactone
- Carboxylic acid ester
- Oxacycle
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organoheterocyclic compound
- Alcohol
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Carbonyl group
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0ufr-4791000000-7c333cef2728f45b278c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0fh9-6891000000-63a42f621abe29558341 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00b9-9067000000-8c18c4d2c7eb2e24144a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-9070000000-bc023871fd50d0a4dcd5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00bc-9310000000-3c8d3a755fa94974bef1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-1059000000-39d5e2d8f3a1bac69bdf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00b9-4393000000-5f04cfdd3b9c108ec083 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00dr-9800000000-b24fbbce7a819e18e2a0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0029000000-2e077979730611444d85 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fb9-0091000000-f9df822c4914cb20c3f0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udi-2970000000-65b43a28e91b908f1964 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0fb9-0069000000-a245733346783b3675ba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fb9-0097000000-d93229107e28be4258b8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ufr-0390000000-37fe8a2f41d25a30ae2e | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036152 |
|---|
| FooDB ID | FDB015003 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00013000 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 10424698 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 13944248 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|