| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 23:53:55 UTC |
|---|
| Update Date | 2016-11-09 01:18:30 UTC |
|---|
| Accession Number | CHEM027670 |
|---|
| Identification |
|---|
| Common Name | Feruloyl-2-hydroxyputrescine |
|---|
| Class | Small Molecule |
|---|
| Description | Feruloyl-2-hydroxyputrescine is found in cereals and cereal products. Feruloyl-2-hydroxyputrescine is an alkaloid from Triticum aestivum (wheat). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
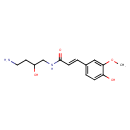
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (2E)-N-(4-Amino-2-hydroxybutyl)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enimidate | HMDB |
|
|---|
| Chemical Formula | C14H20N2O4 |
|---|
| Average Molecular Mass | 280.320 g/mol |
|---|
| Monoisotopic Mass | 280.142 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (2E)-N-(4-amino-2-hydroxybutyl)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enamide |
|---|
| Traditional Name | (2E)-N-(4-amino-2-hydroxybutyl)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enamide |
|---|
| SMILES | COC1=CC(\C=C\C(=O)NCC(O)CCN)=CC=C1O |
|---|
| InChI Identifier | InChI=1S/C14H20N2O4/c1-20-13-8-10(2-4-12(13)18)3-5-14(19)16-9-11(17)6-7-15/h2-5,8,11,17-18H,6-7,9,15H2,1H3,(H,16,19)/b5-3+ |
|---|
| InChI Key | MHXWDKFRPOVGGG-HWKANZROSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as hydroxycinnamic acids and derivatives. Hydroxycinnamic acids and derivatives are compounds containing an cinnamic acid (or a derivative thereof) where the benzene ring is hydroxylated. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Cinnamic acids and derivatives |
|---|
| Sub Class | Hydroxycinnamic acids and derivatives |
|---|
| Direct Parent | Hydroxycinnamic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hydroxycinnamic acid or derivatives
- Cinnamic acid amide
- Methoxyphenol
- Phenoxy compound
- Phenol ether
- Styrene
- Methoxybenzene
- Anisole
- Phenol
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Benzenoid
- Monocyclic benzene moiety
- 1,3-aminoalcohol
- Secondary carboxylic acid amide
- Secondary alcohol
- Carboxamide group
- Amino acid or derivatives
- Ether
- Carboxylic acid derivative
- Organic nitrogen compound
- Primary aliphatic amine
- Organonitrogen compound
- Organooxygen compound
- Primary amine
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Amine
- Organic oxygen compound
- Alcohol
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-001i-9240000000-31c17f7e20834abcb487 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-053r-9122400000-caa430be8333c7340828 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-2390000000-2b2cba862b81515e1c8c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-9530000000-a56b4b9bbda7dca672e3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-9300000000-b6593426df43716f33ce | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0290000000-34d71bbc15745a48b50b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01tc-3960000000-9a28bcd41a5d3ec8d0e5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9700000000-ef50cfe293e7f5f6fe6b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0190000000-4bb3896688505ea02791 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0080-2910000000-004c5c3363eac6463ba4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001d-1900000000-d52c752e1eb4ce6a8ad2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0290000000-778b3abb8d5d47b09809 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01r2-1980000000-d1f020535b5f6a5f6436 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-002k-5910000000-34b2222c34d25cb01d5d | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0033465 |
|---|
| FooDB ID | FDB011507 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35013609 |
|---|
| ChEBI ID | 172311 |
|---|
| PubChem Compound ID | 131751430 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|