| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 23:53:45 UTC |
|---|
| Update Date | 2016-11-09 01:18:30 UTC |
|---|
| Accession Number | CHEM027665 |
|---|
| Identification |
|---|
| Common Name | 4-Hydroxycinnamoylagmatine |
|---|
| Class | Small Molecule |
|---|
| Description | A p-coumaroylagmatine(1+) in which the double bond of the coumaroyl component has E-geochemistry. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
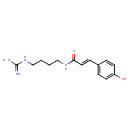
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (e)-Coumaroylagmatine | ChEBI | | (e)-N-(4-Guanidinobutyl)-4-hydroxycinnamamide | ChEBI | | 1-(trans-4'-Hydroxycinnamoylamino)-4-guanidinobutane | ChEBI | | trans-Coumaroylagmatine | ChEBI | | trans-p-Coumaroylagmatine | ChEBI | | N-(4-Guanidinobutyl)-4-hydroxycinnamide | Kegg | | N-(4-Guanidinobutyl)-4-hydroxycinnamamide | Kegg | | cis-P-Coumaroylagmatine | HMDB | | N1-trans-P-Coumaroylagmatine | HMDB |
|
|---|
| Chemical Formula | C14H20N4O2 |
|---|
| Average Molecular Mass | 276.334 g/mol |
|---|
| Monoisotopic Mass | 276.159 g/mol |
|---|
| CAS Registry Number | 7295-86-5 |
|---|
| IUPAC Name | (2E)-N-(4-carbamimidamidobutyl)-3-(4-hydroxyphenyl)prop-2-enamide |
|---|
| Traditional Name | (2E)-N-(4-carbamimidamidobutyl)-3-(4-hydroxyphenyl)prop-2-enamide |
|---|
| SMILES | NC(=N)NCCCCNC(=O)\C=C\C1=CC=C(O)C=C1 |
|---|
| InChI Identifier | InChI=1S/C14H20N4O2/c15-14(16)18-10-2-1-9-17-13(20)8-5-11-3-6-12(19)7-4-11/h3-8,19H,1-2,9-10H2,(H,17,20)(H4,15,16,18)/b8-5+ |
|---|
| InChI Key | AKIHYQWCLCDMMI-VMPITWQZSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as coumaric acids and derivatives. These are aromatic compounds containing Aromatic compounds containing a cinnamic acid moiety (or a derivative thereof) hydroxylated at the C2 (ortho-), C3 (meta-), or C4 (para-) carbon atom of the benzene ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Cinnamic acids and derivatives |
|---|
| Sub Class | Hydroxycinnamic acids and derivatives |
|---|
| Direct Parent | Coumaric acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cinnamic acid amide
- Coumaric acid or derivatives
- Styrene
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Monocyclic benzene moiety
- Benzenoid
- Carboxamide group
- Guanidine
- Secondary carboxylic acid amide
- Carboxylic acid derivative
- Carboximidamide
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-5900000000-dae641a9d8c5b411bb43 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-022d-8692000000-f43d311d6603321aa819 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-1940000000-073108419d02e5ccd9ef | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03fr-5900000000-c7ebd6eb8c81870020b6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-9200000000-b958fc502e80b5e61211 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0059-1290000000-e3ee5ea565154da32314 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a59-7690000000-fe62d436c3fdfc1a6c0b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9200000000-584c6befeed25249f330 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0090000000-41147c37d92a3746a6f4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05po-4790000000-42b3634d1f55c434820d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-3900000000-1b0829f370c10adaa199 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0090000000-83528a2538e0340b9e70 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-1390000000-14620c18029a5c5b18ba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-5900000000-fbb62a538d059fea29b9 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0033460 |
|---|
| FooDB ID | FDB011502 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00028060 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4444279 |
|---|
| ChEBI ID | 86080 |
|---|
| PubChem Compound ID | 5280691 |
|---|
| Kegg Compound ID | C04498 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|