| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 23:53:25 UTC |
|---|
| Update Date | 2016-11-09 01:18:30 UTC |
|---|
| Accession Number | CHEM027658 |
|---|
| Identification |
|---|
| Common Name | Fagomine |
|---|
| Class | Small Molecule |
|---|
| Description | Fagomine is an alkaloid found in the seeds of Castanospermum australe (commonly known as the Black Bean or the Moreton Bay Chestnut) (PMID: 25583438). Castanospermum australe is a large evergreen tree of the legume family native to the east coast of Australia in Queensland and New South Wales, and to the Pacific islands of Vanuatu, New Caledonia, and the island of New Britain (Papua New Guinea). The seeds are poisonous, but become edible when carefully prepared by roasting, cutting up into small pieces, leaching with running water for several days, and pounding into flour (Wikipedia). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
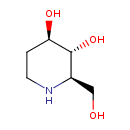
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| D-Fagomine | ChEMBL, HMDB | | Fagomine | MeSH | | 1,2,5-Trideoxy-1,5-imino-D-arabino-hexitol | MeSH, HMDB | | (2R,3R,4R)-2-(Hydroxymethyl)-3,4-piperidinediol | PhytoBank |
|
|---|
| Chemical Formula | C6H13NO3 |
|---|
| Average Molecular Mass | 147.172 g/mol |
|---|
| Monoisotopic Mass | 147.090 g/mol |
|---|
| CAS Registry Number | 53185-12-9 |
|---|
| IUPAC Name | (2R,3R,4R)-2-(hydroxymethyl)piperidine-3,4-diol |
|---|
| Traditional Name | fagomine |
|---|
| SMILES | OCC1NCCC(O)C1O |
|---|
| InChI Identifier | InChI=1S/C6H13NO3/c8-3-4-6(10)5(9)1-2-7-4/h4-10H,1-3H2 |
|---|
| InChI Key | YZNNBIPIQWYLDM-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as piperidines. Piperidines are compounds containing a piperidine ring, which is a saturated aliphatic six-member ring with one nitrogen atom and five carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Piperidines |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Piperidines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Piperidine
- 1,2-aminoalcohol
- Secondary alcohol
- Secondary aliphatic amine
- Azacycle
- Secondary amine
- Organic nitrogen compound
- Hydrocarbon derivative
- Primary alcohol
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Amine
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00ri-9400000000-3b8a5882645861b88c33 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000t-0900000000-1cd0371a246b72560112 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01q9-1900000000-bf1bf8f8fc28cdba567f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03dl-9600000000-eb5b3f84b34a61c5ea29 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-1900000000-d532dc64bdef94e8ec0a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00mn-9700000000-01b152f05c6f3ed1cd4c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9000000000-c81ae5d7d7853799165f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-f139716f16d745072c5a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4j-9400000000-8a791e320b3bb834d1b6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9000000000-54b85f2d100a98c86df4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03e9-0900000000-af1e57e01fc85eeb862f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001j-5900000000-f038cfecd4438da4f325 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9200000000-6fdcd83134b6d7537397 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0033453 |
|---|
| FooDB ID | FDB011491 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00002038 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 65215 |
|---|
| ChEBI ID | 4969 |
|---|
| PubChem Compound ID | 72259 |
|---|
| Kegg Compound ID | C10144 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Kato A, Hirokami Y, Kinami K, Tsuji Y, Miyawaki S, Adachi I, Hollinshead J, Nash RJ, Kiappes JL, Zitzmann N, Cha JK, Molyneux RJ, Fleet GW, Asano N: Isolation and SAR studies of bicyclic iminosugars from Castanospermum australe as glycosidase inhibitors. Phytochemistry. 2015 Mar;111:124-31. doi: 10.1016/j.phytochem.2014.12.011. Epub 2015 Jan 9. | | 2. Yannai, Shmuel. (2004) Dictionary of food compounds with CD-ROM: Additives, flavors, and ingredients. Boca Raton: Chapman & Hall/CRC. |
|
|---|