| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 23:53:08 UTC |
|---|
| Update Date | 2016-11-09 01:18:30 UTC |
|---|
| Accession Number | CHEM027651 |
|---|
| Identification |
|---|
| Common Name | Rutagravine |
|---|
| Class | Small Molecule |
|---|
| Description | Alkaloid from the callus tissue of Ruta graveolens (rue). Rutagravine is found in herbs and spices. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
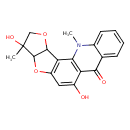
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C19H17NO5 |
|---|
| Average Molecular Mass | 339.342 g/mol |
|---|
| Monoisotopic Mass | 339.111 g/mol |
|---|
| CAS Registry Number | 101330-60-3 |
|---|
| IUPAC Name | 6,11-dihydroxy-6,20-dimethyl-4,8-dioxa-20-azapentacyclo[10.8.0.0²,⁹.0³,⁷.0¹⁴,¹⁹]icosa-1(12),2(9),10,14,16,18-hexaen-13-one |
|---|
| Traditional Name | 6,11-dihydroxy-6,20-dimethyl-4,8-dioxa-20-azapentacyclo[10.8.0.0²,⁹.0³,⁷.0¹⁴,¹⁹]icosa-1(12),2(9),10,14,16,18-hexaen-13-one |
|---|
| SMILES | CN1C2=CC=CC=C2C(=O)C2=C1C1=C(OC3C1OCC3(C)O)C=C2O |
|---|
| InChI Identifier | InChI=1S/C19H17NO5/c1-19(23)8-24-17-14-12(25-18(17)19)7-11(21)13-15(14)20(2)10-6-4-3-5-9(10)16(13)22/h3-7,17-18,21,23H,8H2,1-2H3 |
|---|
| InChI Key | SWALXCKAJQTSAC-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as acridones. These are acridines containing a ketone group attached to the C9 carbon atom of the acridine moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Quinolines and derivatives |
|---|
| Sub Class | Benzoquinolines |
|---|
| Direct Parent | Acridones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Acridone
- Dihydroquinolone
- Dihydroquinoline
- Coumaran
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Pyridine
- Benzenoid
- Vinylogous amide
- Vinylogous acid
- Heteroaromatic compound
- Tetrahydrofuran
- Tertiary alcohol
- Oxacycle
- Azacycle
- Dialkyl ether
- Ether
- Hydrocarbon derivative
- Organonitrogen compound
- Organic oxide
- Organopnictogen compound
- Organooxygen compound
- Organic nitrogen compound
- Organic oxygen compound
- Alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-052f-9075000000-1244bc096e5f3575fd7a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-01bi-1405900000-a0d6661f995064d02fbf | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0019000000-ed52f50c2b07a3d1f971 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-006x-0019000000-2b7ea94dbdd073dfb457 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-1291000000-d9095fe856a3e7305e0e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0009000000-d44b2dffae980b7968fa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0079-0009000000-3b56b5084e958f3f9884 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-1191000000-1cee95f0ff64b4a02461 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0009000000-44ba58366199301e50a3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0098000000-d3e1c79e6464aab68036 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-053i-0059000000-19966651bdec8b6213bc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0009000000-ad69679611f6ad4c80b6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0019000000-edca42c96fe11fcc7e8b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-006x-1196000000-113443014424f72386ab | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0033446 |
|---|
| FooDB ID | FDB011484 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4577337 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 5465778 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|