| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 23:52:48 UTC |
|---|
| Update Date | 2016-11-09 01:18:30 UTC |
|---|
| Accession Number | CHEM027644 |
|---|
| Identification |
|---|
| Common Name | Nigellimine N-oxide |
|---|
| Class | Small Molecule |
|---|
| Description | Nigellimine N-oxide is found in herbs and spices. Minor alkaloid from the seeds of Nigella sativa (blcak cumin). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
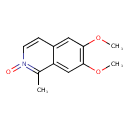
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (2S,3's)-a-Amino-2-carboxy-5-oxo-1-pyrrolidinebutanoate | HMDB | | (2S,3's)-a-Amino-2-carboxy-5-oxo-1-pyrrolidinebutanoic acid | HMDB | | (2S,3's)-alpha-Amino-2-carboxy-5-oxo-1-pyrrolidinebutanoate | HMDB | | (2S,3's)-Α-amino-2-carboxy-5-oxo-1-pyrrolidinebutanoate | HMDB | | (2S,3's)-Α-amino-2-carboxy-5-oxo-1-pyrrolidinebutanoic acid | HMDB |
|
|---|
| Chemical Formula | C12H13NO3 |
|---|
| Average Molecular Mass | 219.237 g/mol |
|---|
| Monoisotopic Mass | 219.090 g/mol |
|---|
| CAS Registry Number | 96562-85-5 |
|---|
| IUPAC Name | 6,7-dimethoxy-1-methyl-2λ⁵-isoquinolin-2-one |
|---|
| Traditional Name | 6,7-dimethoxy-1-methyl-2λ⁵-isoquinolin-2-one |
|---|
| SMILES | COC1=C(OC)C=C2C(C)=N(=O)C=CC2=C1 |
|---|
| InChI Identifier | InChI=1S/C12H13NO3/c1-8-10-7-12(16-3)11(15-2)6-9(10)4-5-13(8)14/h4-7H,1-3H3 |
|---|
| InChI Key | GVQWAJKTFATJEW-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as isoquinolines and derivatives. These are aromatic polycyclic compounds containing an isoquinoline moiety, which consists of a benzene ring fused to a pyridine ring and forming benzo[c]pyridine. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Isoquinolines and derivatives |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Isoquinolines and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Isoquinoline
- Anisole
- Alkyl aryl ether
- Methylpyridine
- Benzenoid
- Pyridinium
- Pyridine
- Heteroaromatic compound
- Azacycle
- Ether
- Organic nitrogen compound
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Organic oxide
- Organopnictogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01ti-0920000000-a70411a7925f58442fc5 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0090000000-04f2f33e189e74a7f296 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0090000000-d4033406756a8e8f17a6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001j-2900000000-bef23815380c5d94afe5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0090000000-ca6b7de448cb829b8a7a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0090000000-57c9c36f3e5050cd9dbe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-1910000000-74fdc16d40f3ceb85bca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0090000000-0b5da4c845aad7d55631 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fk9-0190000000-8f31fa8b6faae4b2ce1e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01rj-0900000000-8e659a4b89b6a655643b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0090000000-9099167b0a94f45e6174 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0390000000-20a1050d6289fd8d84d0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0bua-0900000000-204d03ef57ca987a17a4 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0033436 |
|---|
| FooDB ID | FDB011474 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 26773844 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 14101166 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|