| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 23:51:02 UTC |
|---|
| Update Date | 2016-11-09 01:18:29 UTC |
|---|
| Accession Number | CHEM027608 |
|---|
| Identification |
|---|
| Common Name | C.I. Acid Green 50 |
|---|
| Class | Small Molecule |
|---|
| Description | C.I. Acid Green 50 is a food colourant. Prohibited in Japan, Canada, USA, Norway, Sweden and Finland. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
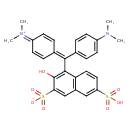
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Acid brilliant green | HMDB | | C.I. 44090 | HMDB | | C.I. FOOD green 4 | HMDB | | e142 | HMDB | | FOOD Green S | HMDB | | Green S | HMDB | | Lissamine green b | HMDB | | N-[4-[[4-(dimethylamino)Phenyl](2-hydroxy-3,6-disulfO-1-naphthalenyl)methylene]-2,5-cyclohexadien-1-ylidene]-1-methylmethanaminium hydroxide inner salt | HMDB | | (5-{[4-(dimethylamino)phenyl][4-(dimethyliminiumyl)cyclohexa-2,5-dien-1-ylidene]methyl}-6-oxo-7-sulfO-2,6-dihydronaphthalen-2-ylidene)(hydroxy)-λ⁶-sulfanoylolic acid | Generator | | (5-{[4-(dimethylamino)phenyl][4-(dimethyliminiumyl)cyclohexa-2,5-dien-1-ylidene]methyl}-6-oxo-7-sulphO-2,6-dihydronaphthalen-2-ylidene)(hydroxy)-λ⁶-sulphanoylolate | Generator | | (5-{[4-(dimethylamino)phenyl][4-(dimethyliminiumyl)cyclohexa-2,5-dien-1-ylidene]methyl}-6-oxo-7-sulphO-2,6-dihydronaphthalen-2-ylidene)(hydroxy)-λ⁶-sulphanoylolic acid | Generator |
|
|---|
| Chemical Formula | C27H26N2O7S2 |
|---|
| Average Molecular Mass | 554.635 g/mol |
|---|
| Monoisotopic Mass | 554.118 g/mol |
|---|
| CAS Registry Number | 25317-10-6 |
|---|
| IUPAC Name | 4-{[4-(dimethylamino)phenyl][4-(dimethyliminiumyl)cyclohexa-2,5-dien-1-ylidene]methyl}-3-hydroxy-7-sulfonaphthalene-2-sulfonate |
|---|
| Traditional Name | 4-{[4-(dimethylamino)phenyl][4-(dimethyliminio)cyclohexa-2,5-dien-1-ylidene]methyl}-3-hydroxy-7-sulfonaphthalene-2-sulfonate |
|---|
| SMILES | CN(C)C1=CC=C(C=C1)C(=C1C=CC(C=C1)=[N+](C)C)C1=C(O)C(=CC2=C1C=CC(=C2)S(O)(=O)=O)S([O-])(=O)=O |
|---|
| InChI Identifier | InChI=1S/C27H26N2O7S2/c1-28(2)20-9-5-17(6-10-20)25(18-7-11-21(12-8-18)29(3)4)26-23-14-13-22(37(31,32)33)15-19(23)16-24(27(26)30)38(34,35)36/h5-16H,1-4H3,(H2,31,32,33,34,35,36) |
|---|
| InChI Key | QEFJYMFUMVHTBR-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as sulfonated stilbenes. These are stilbenes that carry a sulfone group at one or more positions of either benzene rings. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Stilbenes |
|---|
| Sub Class | Sulfonated stilbenes |
|---|
| Direct Parent | Sulfonated stilbenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Sulfonated stilbene
- Naphthalene sulfonate
- 2-naphthalene sulfonic acid or derivatives
- Naphthalene sulfonic acid or derivatives
- 2-naphthalene sulfonate
- 2-naphthol
- Naphthalene
- Arylsulfonic acid or derivatives
- 1-sulfo,2-unsubstituted aromatic compound
- Aniline or substituted anilines
- Dialkylarylamine
- Tertiary aliphatic/aromatic amine
- Benzenoid
- Monocyclic benzene moiety
- Secondary ketimine
- Organic sulfonic acid or derivatives
- Organosulfonic acid or derivatives
- Azomethine
- Sulfonyl
- Organosulfonic acid
- Tertiary amine
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Amine
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Organic zwitterion
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00di-0011960000-ba095700dac7281fa9c4 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0h9r-5010194000-6d06e15711b4c2ffc33f | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("C.I. Acid Green 50,1TMS,#1" TMS) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0000090000-b9295d5013cb712cb90b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0000090000-471198357787b85d9f69 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05o0-9010330000-53ccdba4cc419a3ba698 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0uk9-0000590000-a7024d314ab9b965f011 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fk9-0000970000-e0066d266d92d42c81b1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0f7o-1009300000-2b76bfda60ee262fe6d3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0000090000-27fd80bfa7bdcb9ec6ff | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0aor-0110190000-dedf48fbf291a99bfb06 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f6t-8930010000-eddac4b5897d90113518 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0000390000-5096686c28c005316186 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05di-0000940000-e48077dcd53ad60412fe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0uk9-0143890000-3d9ac09ecce25273800f | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0033390 |
|---|
| FooDB ID | FDB011423 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 82526 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 91394 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|