| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 23:50:26 UTC |
|---|
| Update Date | 2016-11-09 01:18:29 UTC |
|---|
| Accession Number | CHEM027597 |
|---|
| Identification |
|---|
| Common Name | (S)-Nandigerine |
|---|
| Class | Small Molecule |
|---|
| Description | (S)-Nandigerine is found in herbs and spices. (S)-Nandigerine is an alkaloid from Laurus nobilis (bay laurel). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
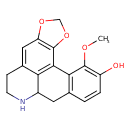
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (+)-Nandigerine | HMDB | | Hernangerine | HMDB | | Nandigerine | HMDB |
|
|---|
| Chemical Formula | C18H17NO4 |
|---|
| Average Molecular Mass | 311.332 g/mol |
|---|
| Monoisotopic Mass | 311.116 g/mol |
|---|
| CAS Registry Number | 31520-97-5 |
|---|
| IUPAC Name | 18-methoxy-3,5-dioxa-11-azapentacyclo[10.7.1.0²,⁶.0⁸,²⁰.0¹⁴,¹⁹]icosa-1(20),2(6),7,14(19),15,17-hexaen-17-ol |
|---|
| Traditional Name | 18-methoxy-3,5-dioxa-11-azapentacyclo[10.7.1.0²,⁶.0⁸,²⁰.0¹⁴,¹⁹]icosa-1(20),2(6),7,14(19),15,17-hexaen-17-ol |
|---|
| SMILES | COC1=C(O)C=CC2=C1C1=C3C(C2)NCCC3=CC2=C1OCO2 |
|---|
| InChI Identifier | InChI=1S/C18H17NO4/c1-21-17-12(20)3-2-9-6-11-14-10(4-5-19-11)7-13-18(23-8-22-13)16(14)15(9)17/h2-3,7,11,19-20H,4-6,8H2,1H3 |
|---|
| InChI Key | CFUKKPQRQGCLAT-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as aporphines. These are quinoline alkaloids containing the dibenzo[de,g]quinoline ring system or a dehydrogenated derivative thereof. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Aporphines |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Aporphines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aporphine
- Benzoquinoline
- Phenanthrene
- 2-naphthol
- Naphthalene
- Tetrahydroisoquinoline
- Quinoline
- Benzodioxole
- Anisole
- 1-hydroxy-2-unsubstituted benzenoid
- Aralkylamine
- Alkyl aryl ether
- Benzenoid
- Organoheterocyclic compound
- Azacycle
- Ether
- Secondary aliphatic amine
- Acetal
- Oxacycle
- Secondary amine
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Amine
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-001j-0090000000-c316f8069ff05049c88e | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-01b9-1039000000-3216986572277372a53a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0129000000-3d9dcb65008d6666ac64 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0196000000-79157dbb750c28dc1521 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0084-0980000000-20dc1359b1775cc0aa99 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0019000000-c37b1f338cdbb6ccf785 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0069000000-f5ddb8357bd02461a43a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01p6-1190000000-086cf3907f446923abdb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0009000000-4631b5d1b4d93faf70b7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0009000000-7f26a29f7533604f9414 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-02au-0191000000-154a5edb70a5ddafd123 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0009000000-43e89ef889a2eb4f124e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03dl-0098000000-895556af222b2f0b68ee | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01pc-0090000000-c7d6452be1f2e4b9f599 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0033362 |
|---|
| FooDB ID | FDB011390 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00027566 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 374109 |
|---|
| ChEBI ID | 174972 |
|---|
| PubChem Compound ID | 422682 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|