| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 23:49:43 UTC |
|---|
| Update Date | 2016-11-09 01:18:29 UTC |
|---|
| Accession Number | CHEM027577 |
|---|
| Identification |
|---|
| Common Name | Mollicellin E |
|---|
| Class | Small Molecule |
|---|
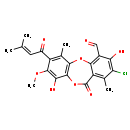
| Description | A member of the class of depsidones that is 11H-dibenzo[b,e][1,4]dioxepine substituted by a chloro group at position 2, hydroxy groups at positions 3 and 9, a methoxy group at position 8, methyl groups at positions 1 and 6, a 3-methylbut-2-enoyl group at position 7, an oxo group at position 11 and a formyl group at position 4. Isolated from Chaetomium brasiliense, it exhibits antimalarial and cytotoxic activities. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C22H19ClO8 |
|---|
| Average Molecular Mass | 446.834 g/mol |
|---|
| Monoisotopic Mass | 446.077 g/mol |
|---|
| CAS Registry Number | 68455-10-7 |
|---|
| IUPAC Name | 13-chloro-7,14-dihydroxy-6-methoxy-4,12-dimethyl-5-(3-methylbut-2-enoyl)-10-oxo-2,9-dioxatricyclo[9.4.0.0³,⁸]pentadeca-1(15),3(8),4,6,11,13-hexaene-15-carbaldehyde |
|---|
| Traditional Name | mollicellin E |
|---|
| SMILES | COC1=C(O)C2=C(OC3=C(C=O)C(O)=C(Cl)C(C)=C3C(=O)O2)C(C)=C1C(=O)C=C(C)C |
|---|
| InChI Identifier | InChI=1S/C22H19ClO8/c1-8(2)6-12(25)13-10(4)18-21(17(27)20(13)29-5)31-22(28)14-9(3)15(23)16(26)11(7-24)19(14)30-18/h6-7,26-27H,1-5H3 |
|---|
| InChI Key | JTGLZOMMRQOKBM-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as depsides and depsidones. These are polycyclic compounds that is either a polyphenolic compound composed of two or more monocyclic aromatic units linked by an ester bond (depside), or a compound containing the depsidone structure (depsidone). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Depsides and depsidones |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Depsides and depsidones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Depsidone
- Diaryl ether
- Anisole
- Aryl ketone
- 1,4-dioxepine
- Alkyl aryl ether
- Dioxepine
- Aryl-aldehyde
- Aryl chloride
- Aryl halide
- Benzenoid
- Alpha,beta-unsaturated ketone
- Acryloyl-group
- Vinylogous acid
- Enone
- Carboxylic acid ester
- Lactone
- Ketone
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Oxacycle
- Organoheterocyclic compound
- Ether
- Organohalogen compound
- Organic oxygen compound
- Aldehyde
- Organochloride
- Organooxygen compound
- Organic oxide
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00lr-1415900000-ea2bdb044bb2f1a08d1f | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0a6r-2070090000-2a988d39ce3e70ba25b3 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-1001900000-fd4a6f406f35c3c3801f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000w-7134900000-bc5c672574beaed49fe0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-029t-9831000000-362ed51b0a33a6fae288 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-1100900000-d4b9858562e2ac770ebb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05mk-4221900000-efd0c1d26de80cad45a5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ac3-5910000000-477e730e33d69703e87e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0002900000-c6ff98f88741a74c4a3b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01pk-0009300000-914c33ba711aae734d9c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0w34-2139100000-034eafdf15b25559fe13 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4j-0001900000-ab41186e4631a24589c8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0005-0006900000-c8f9ab2fc3f2483f0191 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-02fx-2009200000-c1be572ba16c6f569433 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0033342 |
|---|
| FooDB ID | FDB011370 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00048002 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 45523 |
|---|
| ChEBI ID | 68803 |
|---|
| PubChem Compound ID | 50201 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|