| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 23:49:37 UTC |
|---|
| Update Date | 2016-11-09 01:18:29 UTC |
|---|
| Accession Number | CHEM027574 |
|---|
| Identification |
|---|
| Common Name | Mollicellin F |
|---|
| Class | Small Molecule |
|---|
| Description | A member of the class of depsidones that is 3,4-dihydro-H,11H-chromeno[6,7-b][1,4]benzodioxepine substituted by a chloro group at position 9, hydroxy groups at positions 8 and 13, methyl groups at positions 2, 2, 5 and 10, oxo groups at positions 4 and 11 and a formyl group at position 7. Isolated from Chaetomium brasiliense, it exhibits cytotoxic activity. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
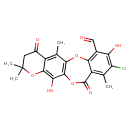
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Pentanoic acid, 4-oxo-, strontium salt | HMDB |
|
|---|
| Chemical Formula | C21H17ClO8 |
|---|
| Average Molecular Mass | 432.808 g/mol |
|---|
| Monoisotopic Mass | 432.061 g/mol |
|---|
| CAS Registry Number | 68455-12-9 |
|---|
| IUPAC Name | 6-chloro-5,12-dihydroxy-7,15,15,19-tetramethyl-9,17-dioxo-2,10,14-trioxatetracyclo[9.8.0.0³,⁸.0¹³,¹⁸]nonadeca-1(11),3,5,7,12,18-hexaene-4-carbaldehyde |
|---|
| Traditional Name | 6-chloro-5,12-dihydroxy-7,15,15,19-tetramethyl-9,17-dioxo-2,10,14-trioxatetracyclo[9.8.0.0³,⁸.0¹³,¹⁸]nonadeca-1(11),3,5,7,12,18-hexaene-4-carbaldehyde |
|---|
| SMILES | CC1=C2C(OC3=C(OC2=O)C(O)=C2OC(C)(C)CC(=O)C2=C3C)=C(C=O)C(O)=C1Cl |
|---|
| InChI Identifier | InChI=1S/C21H17ClO8/c1-7-12-17(9(6-23)14(25)13(7)22)28-16-8(2)11-10(24)5-21(3,4)30-18(11)15(26)19(16)29-20(12)27/h6,25-26H,5H2,1-4H3 |
|---|
| InChI Key | BUWVABSQGVRXOI-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 2,2-dimethyl-1-benzopyrans. These are organic compounds containing a 1-benzopyran moiety that carries two methyl groups at the 2-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzopyrans |
|---|
| Sub Class | 1-benzopyrans |
|---|
| Direct Parent | 2,2-dimethyl-1-benzopyrans |
|---|
| Alternative Parents | |
|---|
| Substituents | - 2,2-dimethyl-1-benzopyran
- Chromone
- Diaryl ether
- Aryl alkyl ketone
- Aryl ketone
- 1,4-dioxepine
- Alkyl aryl ether
- Aryl-aldehyde
- Dioxepine
- Aryl chloride
- Aryl halide
- Benzenoid
- Vinylogous acid
- Carboxylic acid ester
- Ketone
- Lactone
- Ether
- Carboxylic acid derivative
- Oxacycle
- Monocarboxylic acid or derivatives
- Organohalogen compound
- Organochloride
- Organooxygen compound
- Organic oxygen compound
- Hydrocarbon derivative
- Aldehyde
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0udr-0272900000-8bde5400d8f7549baeb8 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-08fu-2263290000-da85a5501747eca4b50a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0002900000-d8fdc822d6ed5d0fac54 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00lr-2248900000-71d5562321f833d7d1df | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-07bb-9821000000-867dd505d919f3081b7e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0100900000-cb87b0d8639d51fcb9f7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0f89-0333900000-641eb82106b134f0fa53 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a6r-9710000000-091507a23cbb8805f933 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0000900000-6c62e6c46797ddea0db6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0002900000-48bd83fe01fb96b4af57 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0iki-2297100000-7d230b69012f483271c7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0000900000-79c2d1b5dd64b5becf89 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-003r-0009800000-3a57a0edc51c3db98b64 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03fr-2019100000-bc6ad363f4c7f9c1fd23 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0033338 |
|---|
| FooDB ID | FDB011366 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00048003 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 134713 |
|---|
| ChEBI ID | 68721 |
|---|
| PubChem Compound ID | 119490 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|