| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 23:49:34 UTC |
|---|
| Update Date | 2016-11-09 01:18:29 UTC |
|---|
| Accession Number | CHEM027573 |
|---|
| Identification |
|---|
| Common Name | Fusarin C |
|---|
| Class | Small Molecule |
|---|
| Description | Fusarin C is produced by many Fusarium species in infected corn. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
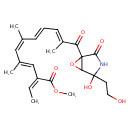
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Methyl (2Z,5Z,7E,9E)-11-[2,4-dihydroxy-4-(2-hydroxyethyl)-6-oxa-3-azabicyclo[3.1.0]hex-2-en-1-yl]-2-ethylidene-4,6,10-trimethyl-11-oxoundeca-3,5,7,9-tetraenoic acid | HMDB |
|
|---|
| Chemical Formula | C23H29NO7 |
|---|
| Average Molecular Mass | 431.479 g/mol |
|---|
| Monoisotopic Mass | 431.194 g/mol |
|---|
| CAS Registry Number | 79748-81-5 |
|---|
| IUPAC Name | methyl (2Z,3E,5Z,7E,9E)-2-ethylidene-11-[4-hydroxy-4-(2-hydroxyethyl)-2-oxo-6-oxa-3-azabicyclo[3.1.0]hexan-1-yl]-4,6,10-trimethyl-11-oxoundeca-3,5,7,9-tetraenoate |
|---|
| Traditional Name | methyl (2Z,3E,5Z,7E,9E)-2-ethylidene-11-[4-hydroxy-4-(2-hydroxyethyl)-2-oxo-6-oxa-3-azabicyclo[3.1.0]hexan-1-yl]-4,6,10-trimethyl-11-oxoundeca-3,5,7,9-tetraenoate |
|---|
| SMILES | COC(=O)\C(=C/C)\C=C(/C)\C=C(\C)/C=C/C=C(\C)C(=O)C12OC1C(O)(CCO)NC2=O |
|---|
| InChI Identifier | InChI=1S/C23H29NO7/c1-6-17(19(27)30-5)13-15(3)12-14(2)8-7-9-16(4)18(26)23-20(31-23)22(29,10-11-25)24-21(23)28/h6-9,12-13,20,25,29H,10-11H2,1-5H3,(H,24,28)/b8-7+,14-12-,15-13+,16-9+,17-6- |
|---|
| InChI Key | FZFYFSUIOSWLHW-OFIJGTIXSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as sesquiterpenoids. These are terpenes with three consecutive isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Sesquiterpenoids |
|---|
| Direct Parent | Sesquiterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Sesquiterpenoid
- Farsesane sesquiterpenoid
- Fatty acid ester
- Oxazinane
- Pyrrolidone
- 2-pyrrolidone
- Alpha-branched alpha,beta-unsaturated-ketone
- Fatty acyl
- Morpholine
- Acryloyl-group
- Pyrrolidine
- Enone
- Methyl ester
- Enoate ester
- Alpha,beta-unsaturated ketone
- Alpha,beta-unsaturated carboxylic ester
- Carboxylic acid ester
- Secondary carboxylic acid amide
- Carboxamide group
- Ketone
- Lactam
- Alkanolamine
- Carboxylic acid derivative
- Dialkyl ether
- Oxirane
- Ether
- Oxacycle
- Azacycle
- Monocarboxylic acid or derivatives
- Organoheterocyclic compound
- Organic nitrogen compound
- Organic oxide
- Carbonyl group
- Hydrocarbon derivative
- Organic oxygen compound
- Alcohol
- Organopnictogen compound
- Organonitrogen compound
- Organooxygen compound
- Primary alcohol
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-03y3-6193500000-609e5116adf462a44862 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-044i-3490140000-d3fa71be0f7b671e5762 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03e9-1222900000-02375304048ebb65837b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-9581100000-e211675ca44508b6945a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-8940000000-e0ef8e52effcb84deae8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-053r-0912600000-e41b93b5330cdb424f04 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0037-2926400000-494acf2d211bc00249fb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-cb310f8645147a361c7d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00yi-0119300000-ddfbaedbffea16b02679 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0830-1956000000-2340cd2222740da1b0ce | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-2920000000-b594e07cce45fe4aa556 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00kb-0109100000-f15cd617e27cae07dee8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-3429000000-63f2597cf580d2105254 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0kbf-3829000000-eb18b15ed92cb7346ccd | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0033337 |
|---|
| FooDB ID | FDB011364 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00016336 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Fusarin |
|---|
| Chemspider ID | 35013595 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131751413 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|