| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 23:45:04 UTC |
|---|
| Update Date | 2016-11-09 01:18:27 UTC |
|---|
| Accession Number | CHEM027457 |
|---|
| Identification |
|---|
| Common Name | (1R,2S,4R,5S)-2,5-Fenchanediol 2-O-b-D-glucoside |
|---|
| Class | Small Molecule |
|---|
| Description | (1R,2S,4R,5S)-2,5-Fenchanediol 2-O-b-D-glucoside is found in herbs and spices. (1R,2S,4R,5S)-2,5-Fenchanediol 2-O-b-D-glucoside is a constituent of Foeniculum vulgare (fennel). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
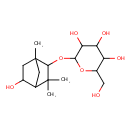
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C16H28O7 |
|---|
| Average Molecular Mass | 332.389 g/mol |
|---|
| Monoisotopic Mass | 332.184 g/mol |
|---|
| CAS Registry Number | 217960-83-3 |
|---|
| IUPAC Name | 2-({5-hydroxy-1,3,3-trimethylbicyclo[2.2.1]heptan-2-yl}oxy)-6-(hydroxymethyl)oxane-3,4,5-triol |
|---|
| Traditional Name | 2-({5-hydroxy-1,3,3-trimethylbicyclo[2.2.1]heptan-2-yl}oxy)-6-(hydroxymethyl)oxane-3,4,5-triol |
|---|
| SMILES | CC1(C)C2CC(C)(CC2O)C1OC1OC(CO)C(O)C(O)C1O |
|---|
| InChI Identifier | InChI=1S/C16H28O7/c1-15(2)7-4-16(3,5-8(7)18)14(15)23-13-12(21)11(20)10(19)9(6-17)22-13/h7-14,17-21H,4-6H2,1-3H3 |
|---|
| InChI Key | LDPSEQUXUZQNEM-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as terpene glycosides. These are prenol lipids containing a carbohydrate moiety glycosidically bound to a terpene backbone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Terpene glycosides |
|---|
| Direct Parent | Terpene glycosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Terpene glycoside
- Hexose monosaccharide
- Glycosyl compound
- O-glycosyl compound
- Bicyclic monoterpenoid
- Fenchane monoterpenoid
- Norbornane monoterpenoid
- Monoterpenoid
- Monosaccharide
- Oxane
- Cyclic alcohol
- Secondary alcohol
- Polyol
- Organoheterocyclic compound
- Oxacycle
- Acetal
- Primary alcohol
- Hydrocarbon derivative
- Organic oxygen compound
- Alcohol
- Organooxygen compound
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0o6s-9235000000-80b85867dfe9905504f6 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (5 TMS) - 70eV, Positive | splash10-004i-1210009000-b5f641fc14aab8817670 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0v59-0905000000-2e8e46a6141f392246bc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uk9-0900000000-7f5dc57bd6abb5353755 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-1900000000-79b9e7582f5ed61b6f02 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0fsi-1918000000-ec3f3cc9c525da95ecc4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0uxr-0901000000-d0b86b9634ee691e7d0d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0gbc-4900000000-8fc183ee627b2e273109 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0009000000-f4dfcd848b7c9cd0e4da | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00lr-5819000000-d271ccd59ec9046594cd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9300000000-ad4e6ca75f888e642148 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uyi-0719000000-d100b22d73af8991264b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001r-2669000000-38d28469242c587bcc79 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-055e-9700000000-1046e0744b70b454c3d1 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0033221 |
|---|
| FooDB ID | FDB011235 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 174507 |
|---|
| PubChem Compound ID | 85232976 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|