| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 23:42:41 UTC |
|---|
| Update Date | 2016-11-09 01:18:27 UTC |
|---|
| Accession Number | CHEM027398 |
|---|
| Identification |
|---|
| Common Name | Melilotocarpan A |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
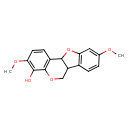
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4-Hydroxy-3,9-dimethoxypterocarpan | HMDB | | 4-Hydroxyhomopterocarpin | HMDB |
|
|---|
| Chemical Formula | C17H16O5 |
|---|
| Average Molecular Mass | 300.306 g/mol |
|---|
| Monoisotopic Mass | 300.100 g/mol |
|---|
| CAS Registry Number | 61135-95-3 |
|---|
| IUPAC Name | 5,14-dimethoxy-8,17-dioxatetracyclo[8.7.0.0²,⁷.0¹¹,¹⁶]heptadeca-2(7),3,5,11(16),12,14-hexaen-6-ol |
|---|
| Traditional Name | 5,14-dimethoxy-8,17-dioxatetracyclo[8.7.0.0²,⁷.0¹¹,¹⁶]heptadeca-2(7),3,5,11(16),12,14-hexaen-6-ol |
|---|
| SMILES | COC1=CC2=C(C=C1)C1COC3=C(C=CC(OC)=C3O)C1O2 |
|---|
| InChI Identifier | InChI=1S/C17H16O5/c1-19-9-3-4-10-12-8-21-17-11(16(12)22-14(10)7-9)5-6-13(20-2)15(17)18/h3-7,12,16,18H,8H2,1-2H3 |
|---|
| InChI Key | YHSXPHNOIMTWTH-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pterocarpans. These are benzo-pyrano-furano-benzene compounds, containing the 6H-[1]benzofuro[3,2-c]chromene skeleton. They are derivatives of isoflavonoids. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Isoflavonoids |
|---|
| Sub Class | Furanoisoflavonoids |
|---|
| Direct Parent | Pterocarpans |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pterocarpan
- Isoflavanol
- Isoflavan
- Chromane
- 1-benzopyran
- Benzopyran
- Coumaran
- Benzofuran
- Anisole
- 1-hydroxy-4-unsubstituted benzenoid
- Alkyl aryl ether
- Benzenoid
- Oxacycle
- Organoheterocyclic compound
- Ether
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0079-0890000000-e84db6797a37ac28c1fd | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0ab9-2139000000-b152124ba1f48f5aa04e | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udr-0619000000-844721337747e7ab2e31 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udr-0946000000-f90a23ca127ad2599dd6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-053r-8900000000-a1f903592c7be3fb4ec5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0090000000-e9f51ee649a094f87353 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0090000000-3d1e2dad59533c854511 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0nu0-2960000000-730f14734fd6a8133b65 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0009000000-7fd5921050e52686aed7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0509000000-f9212a7266c0db474ac3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-1920000000-6fe3ee2d8ed32e90a582 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0090000000-c8e04b941de97dd002f4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0090000000-7f305f9d4ab17528533f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kf-1290000000-49f30f7fe366c6aad6be | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00002536 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 391125 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 442792 |
|---|
| Kegg Compound ID | C10461 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|