| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 23:39:22 UTC |
|---|
| Update Date | 2016-11-09 01:18:26 UTC |
|---|
| Accession Number | CHEM027307 |
|---|
| Identification |
|---|
| Common Name | Thalicpureine |
|---|
| Class | Small Molecule |
|---|
| Description | A phenanthrene substituted by a 2-(methylamino)ethyl group at position 1 and by methoxy groups at positions 2,3,4,6 and 7, respectively. It is a plant metabolite isolated from Annona purpurea and Fagonia olivieri. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
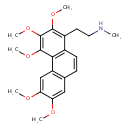
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Methyl[2-(2,3,4,6,7-pentamethoxyphenanthren-1-yl)ethyl]amine | ChEBI | | 2,3,4,6,7-Pentamethoxy-1-(2-methylaminoethyl)phenanthrene | HMDB | | 2,3,4,6,7-Pentamethoxy-N-methyl-1-phenanthrenemethanamine, 9ci | HMDB |
|
|---|
| Chemical Formula | C22H27NO5 |
|---|
| Average Molecular Mass | 385.454 g/mol |
|---|
| Monoisotopic Mass | 385.189 g/mol |
|---|
| CAS Registry Number | 218900-91-5 |
|---|
| IUPAC Name | methyl[2-(2,3,4,6,7-pentamethoxyphenanthren-1-yl)ethyl]amine |
|---|
| Traditional Name | methyl[2-(2,3,4,6,7-pentamethoxyphenanthren-1-yl)ethyl]amine |
|---|
| SMILES | CNCCC1=C(OC)C(OC)=C(OC)C2=C1C=CC1=C2C=C(OC)C(OC)=C1 |
|---|
| InChI Identifier | InChI=1S/C22H27NO5/c1-23-10-9-15-14-8-7-13-11-17(24-2)18(25-3)12-16(13)19(14)21(27-5)22(28-6)20(15)26-4/h7-8,11-12,23H,9-10H2,1-6H3 |
|---|
| InChI Key | VMIFHEUVQQHIOK-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 6,6a-secoaporphines. These are alkaloids with a structure that contains an aminoethylphenanthrene moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | 6,6a-secoaporphines |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | 6,6a-secoaporphines |
|---|
| Alternative Parents | |
|---|
| Substituents | - 6,6a-secoaporphine
- Phenanthrene
- Naphthalene
- Phenethylamine
- Anisole
- Alkyl aryl ether
- Aralkylamine
- Benzenoid
- Ether
- Secondary aliphatic amine
- Secondary amine
- Organic nitrogen compound
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Amine
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9007000000-d9c5df17b512fe18037d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0009000000-87103a8f5035e8450ed6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4r-1009000000-9b6e7119db6a85dd724b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-2097000000-9e72a9ae19b846bc04a2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0009000000-b1742f327eed3d40174c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00lr-0009000000-9b4ad1971cb7d64a9b6a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001u-0092000000-572e8d986fda9ff8e683 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0009000000-583d928efffb1e6fd5fb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ff9-0009000000-aa7154bf5407ad463723 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-0049000000-6a0ba99249fa2fb1eb41 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4r-0009000000-95ecce977dd133a2cb82 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0009000000-e8238f32fa08656af6bc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03fr-0049000000-0c08ab14a9d198cb91c2 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0033032 |
|---|
| FooDB ID | FDB011024 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00050253 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 8605618 |
|---|
| ChEBI ID | 143869 |
|---|
| PubChem Compound ID | 10430190 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|