| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 23:32:42 UTC |
|---|
| Update Date | 2016-11-09 01:18:24 UTC |
|---|
| Accession Number | CHEM027169 |
|---|
| Identification |
|---|
| Common Name | 2-(Ethylamino)-4,5-dihydroxybenzamide |
|---|
| Class | Small Molecule |
|---|
| Description | 2-(Ethylamino)-4,5-dihydroxybenzamide is found in herbs and spices. 2-(Ethylamino)-4,5-dihydroxybenzamide is an alkaloid from Piper nigrum (pepper). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
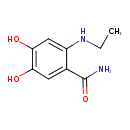
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-(ethylamino)-4,5-Dihydroxybenzamide, 9ci | HMDB | | 2-(Ethylamino)-4,5-dihydroxybenzene-1-carboximidate | Generator |
|
|---|
| Chemical Formula | C9H12N2O3 |
|---|
| Average Molecular Mass | 196.203 g/mol |
|---|
| Monoisotopic Mass | 196.085 g/mol |
|---|
| CAS Registry Number | 127793-87-7 |
|---|
| IUPAC Name | 2-(ethylamino)-4,5-dihydroxybenzamide |
|---|
| Traditional Name | 2-(ethylamino)-4,5-dihydroxybenzamide |
|---|
| SMILES | CCNC1=CC(O)=C(O)C=C1C(N)=O |
|---|
| InChI Identifier | InChI=1S/C9H12N2O3/c1-2-11-6-4-8(13)7(12)3-5(6)9(10)14/h3-4,11-13H,2H2,1H3,(H2,10,14) |
|---|
| InChI Key | VAWDXOCBSZJIEL-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as anthranilamides. These are aromatic compound containing a benzene carboxamide moiety that carries an amine group at the 2-position of the benzene ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzoic acids and derivatives |
|---|
| Direct Parent | Anthranilamides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aminobenzamide
- Anthranilamide
- Aminobenzoic acid or derivatives
- 2-aminobenzamide
- P-aminophenol
- M-aminophenol
- Aminophenol
- Benzoyl
- Catechol
- Phenylalkylamine
- Aniline or substituted anilines
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Secondary aliphatic/aromatic amine
- Vinylogous amide
- Amino acid or derivatives
- Carboxamide group
- Primary carboxylic acid amide
- Carboxylic acid derivative
- Secondary amine
- Organonitrogen compound
- Hydrocarbon derivative
- Amine
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organooxygen compound
- Organic nitrogen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0fus-0900000000-6359e9732da7cb7555f9 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00b9-5098000000-01ee44420257a9561b00 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0900000000-cb4008f18cf4aaf50b09 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ue9-0900000000-5867a53345dd89c2aef1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0kna-4900000000-6ec0a1cb769cda72bdd0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-9f71b2740c0382a9b82f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udj-2900000000-84db2f2d698d972ea641 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9300000000-668d3b1cad21b18670f6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-3ea470ce2c140d3798a4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-1900000000-a3fabbeaf38e7b1f0ea7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9100000000-e05adf90740cb14aa0cb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0900000000-5d9aa7bfa97a880b4afe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ue9-0900000000-544befa69147a58dbd4a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05fu-9800000000-73b24664aab8c2492538 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0032852 |
|---|
| FooDB ID | FDB010829 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00056781 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 8505756 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 10330295 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|