| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 23:31:09 UTC |
|---|
| Update Date | 2016-11-09 01:18:24 UTC |
|---|
| Accession Number | CHEM027133 |
|---|
| Identification |
|---|
| Common Name | Isofucosterol 3-O-[6-O-Octadecanoyl-b-D-glucopyranoside] |
|---|
| Class | Small Molecule |
|---|
| Description | Constituent of Lycopersicon esculentum (tomato). Isofucosterol 3-O-[6-O-Octadecanoyl-b-D-glucopyranoside] is found in garden tomato. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
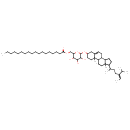
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| [6-({2,15-dimethyl-14-[(5E)-5-(propan-2-yl)hept-5-en-2-yl]tetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-7-en-5-yl}oxy)-3,4,5-trihydroxyoxan-2-yl]methyl octadecanoic acid | Generator |
|
|---|
| Chemical Formula | C53H92O7 |
|---|
| Average Molecular Mass | 841.293 g/mol |
|---|
| Monoisotopic Mass | 840.684 g/mol |
|---|
| CAS Registry Number | 209903-44-6 |
|---|
| IUPAC Name | [6-({2,15-dimethyl-14-[(5E)-5-(propan-2-yl)hept-5-en-2-yl]tetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-7-en-5-yl}oxy)-3,4,5-trihydroxyoxan-2-yl]methyl octadecanoate |
|---|
| Traditional Name | [3,4,5-trihydroxy-6-({14-[(5E)-5-isopropylhept-5-en-2-yl]-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-7-en-5-yl}oxy)oxan-2-yl]methyl octadecanoate |
|---|
| SMILES | CCCCCCCCCCCCCCCCCC(=O)OCC1OC(OC2CCC3(C)C4CCC5(C)C(CCC5C4CC=C3C2)C(C)CC\C(=C/C)C(C)C)C(O)C(O)C1O |
|---|
| InChI Identifier | InChI=1S/C53H92O7/c1-8-10-11-12-13-14-15-16-17-18-19-20-21-22-23-24-47(54)58-36-46-48(55)49(56)50(57)51(60-46)59-41-31-33-52(6)40(35-41)27-28-42-44-30-29-43(53(44,7)34-32-45(42)52)38(5)25-26-39(9-2)37(3)4/h9,27,37-38,41-46,48-51,55-57H,8,10-26,28-36H2,1-7H3/b39-9+ |
|---|
| InChI Key | HPKBXXRAYSTIAI-DNSYQMBRSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as stigmastanes and derivatives. These are sterol lipids with a structure based on the stigmastane skeleton, which consists of a cholestane moiety bearing an ethyl group at the carbon atom C24. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Stigmastanes and derivatives |
|---|
| Direct Parent | Stigmastanes and derivatives |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | Not Available |
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03dj-1068930170-e5591aec2f2557f29308 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03dj-4268900000-ceb614442d3e78f6a9de | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0292-6297301100-7a9efbc72722653b12d4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-02ar-0092410040-918cfb3e31bd3bd06980 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03e9-0092600000-6c69609b9bed9f1f476d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03ea-4087900000-13aa9c7b04367c172b38 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0000300090-16b1a5d55aea6205903a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01p9-1011630090-b5a7e0f20cc8333d4213 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0cea-6044901000-c809d047f4a42f278ad0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0111200290-474cd24380a00d5d3319 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01ow-9057120020-c15bd909991957a5f01f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a5c-9220000000-f2310492c343dcbe1959 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0032812 |
|---|
| FooDB ID | FDB010786 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131751318 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|