| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 23:30:37 UTC |
|---|
| Update Date | 2016-11-09 01:18:24 UTC |
|---|
| Accession Number | CHEM027118 |
|---|
| Identification |
|---|
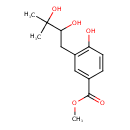
| Common Name | Methyl 3-(2,3-dihydroxy-3-methylbutyl)-4-hydroxybenzoate |
|---|
| Class | Small Molecule |
|---|
| Description | Methyl 3-(2,3-dihydroxy-3-methylbutyl)-4-hydroxybenzoate is a constituent of the famine food Pandanus odoratissimus. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Methyl 3-(2,3-dihydroxy-3-methylbutyl)-4-hydroxybenzoic acid | Generator | | Hostmaniane | HMDB |
|
|---|
| Chemical Formula | C13H18O5 |
|---|
| Average Molecular Mass | 254.279 g/mol |
|---|
| Monoisotopic Mass | 254.115 g/mol |
|---|
| CAS Registry Number | 117176-70-2 |
|---|
| IUPAC Name | methyl 3-(2,3-dihydroxy-3-methylbutyl)-4-hydroxybenzoate |
|---|
| Traditional Name | methyl 3-(2,3-dihydroxy-3-methylbutyl)-4-hydroxybenzoate |
|---|
| SMILES | COC(=O)C1=CC(CC(O)C(C)(C)O)=C(O)C=C1 |
|---|
| InChI Identifier | InChI=1S/C13H18O5/c1-13(2,17)11(15)7-9-6-8(12(16)18-3)4-5-10(9)14/h4-6,11,14-15,17H,7H2,1-3H3 |
|---|
| InChI Key | ABFHVQPVDSMGNR-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as p-hydroxybenzoic acid alkyl esters. These are aromatic compounds containing a benzoic acid, which is esterified with an alkyl group and para-substituted with a hydroxyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzoic acids and derivatives |
|---|
| Direct Parent | p-Hydroxybenzoic acid alkyl esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - P-hydroxybenzoic acid alkyl ester
- Benzoyl
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Methyl ester
- Tertiary alcohol
- 1,2-diol
- Carboxylic acid ester
- Secondary alcohol
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Hydrocarbon derivative
- Organic oxygen compound
- Organic oxide
- Alcohol
- Organooxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-9520000000-52b99f574b1a3506d972 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-053r-4946800000-969db272b3cab7aa23e7 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ap0-0490000000-2cad2044f7dc7f37a1a0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014r-4940000000-9edd345e5afb2f287377 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-002r-2900000000-8ef12e25725fadc5f49a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-1290000000-7c4515ad9824442f8d90 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udr-2890000000-39a849726c1ed7a019f5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00ri-9810000000-f5ea79b4cf8a58a6410b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00lr-0910000000-81051c451d1da423e0c9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a59-1900000000-c9ff46b42eb787805c4b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ufs-1910000000-8951fbfd1cc885bb1c62 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0490000000-4638b7bea81c9458332c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ap0-1940000000-da5e89bced6bd1f90c1e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-3910000000-831f621d377a377b5888 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0032796 |
|---|
| FooDB ID | FDB010768 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00056545 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 9359446 |
|---|
| ChEBI ID | 138773 |
|---|
| PubChem Compound ID | 11184361 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|